Chapter: 08. Some Properties Of Water
Some Properties of Water
Chapter Overview
This chapter will help you understand more about water, its different forms, how it changes from one form to another, how substances dissolve in it, and why some objects float while others sink.
PHYSICAL PROPERTIES OF WATER
Water is all around us and covers most of our planet. When you look at a globe, the large blue areas show where water is present.
Groundwater (underground) Atmosphere (as water vapour) Water exists in three different forms, and all of these forms take up space.
Water as a Solid: Ice
Description: Ice is frozen water. Characteristics: It has a fixed shape and a fixed size. Water as a Liquid: Water
Description: This is the most common form of water we see. It feels wet and can flow. Uses: We use liquid water for many daily activities: Characteristics: It does not have a fixed shape or size. It always takes the shape of the container it is in. Water as a Gas: Water Vapour
Description: Water vapour is water in its gaseous form and is present all around us, even if we can’t always see it. It does not have a fixed shape or size. It spreads out to fill all the available space. CHANGE OF FORMS OF WATER
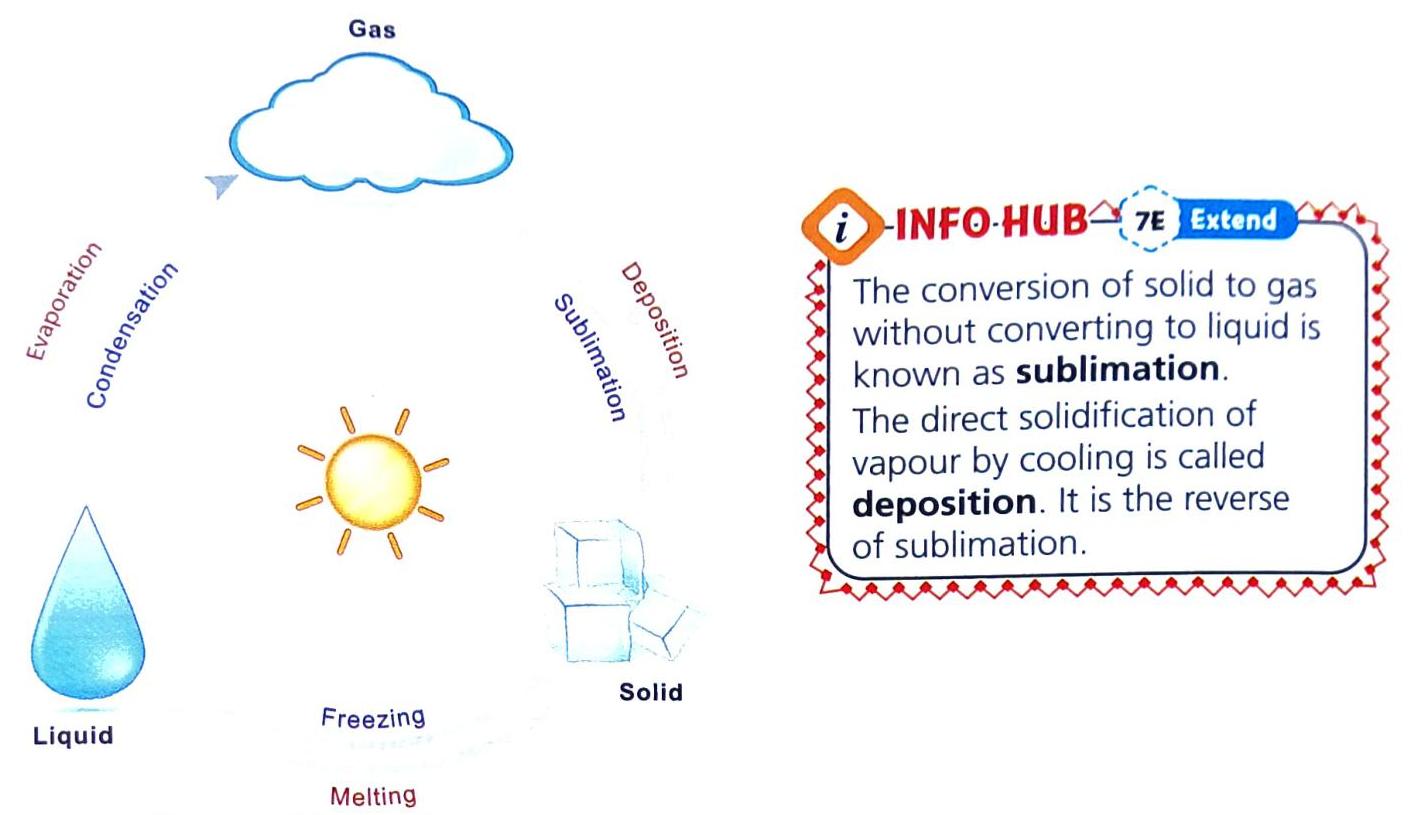
Water can transform from one form to another, depending on temperature changes (heating or cooling).
Solid to Liquid: Melting
Process: A solid changes into a liquid. Cause: Occurs on heating. Example: Ice (solid) melts into water (liquid) when heated. Liquid to Gas: Evaporation
Process: A liquid changes into vapours (gaseous state). Cause: Occurs on heating. Example: When water is heated, steam (water vapour) comes out. This is due to water evaporating into water vapour. Gas to Liquid: Condensation
Process: A gas changes into a liquid. Cause: Occurs on cooling. Example: When you put ice in a glass, water droplets form on the outside surface of the glass. This happens because water vapour in the air cools down and turns back into liquid water when it touches the cold glass. Liquid to Solid: Solidification (Freezing)
Process: A liquid changes into a solid. Cause: Occurs when exposed to freezing temperatures (cooling). Example: Pouring water into an ice tray and putting it in the freezer turns the water (liquid) into ice (solid). Relationship: Freezing is the opposite (reverse) of melting. Beyond Basic Changes: Sublimation and Deposition
Sublimation: This is a special process where a solid changes directly into a gas without first becoming a liquid. Deposition: This is the opposite of sublimation, where a gas changes directly into a solid without first becoming a liquid. WATER AND WATER SOLUTIONS
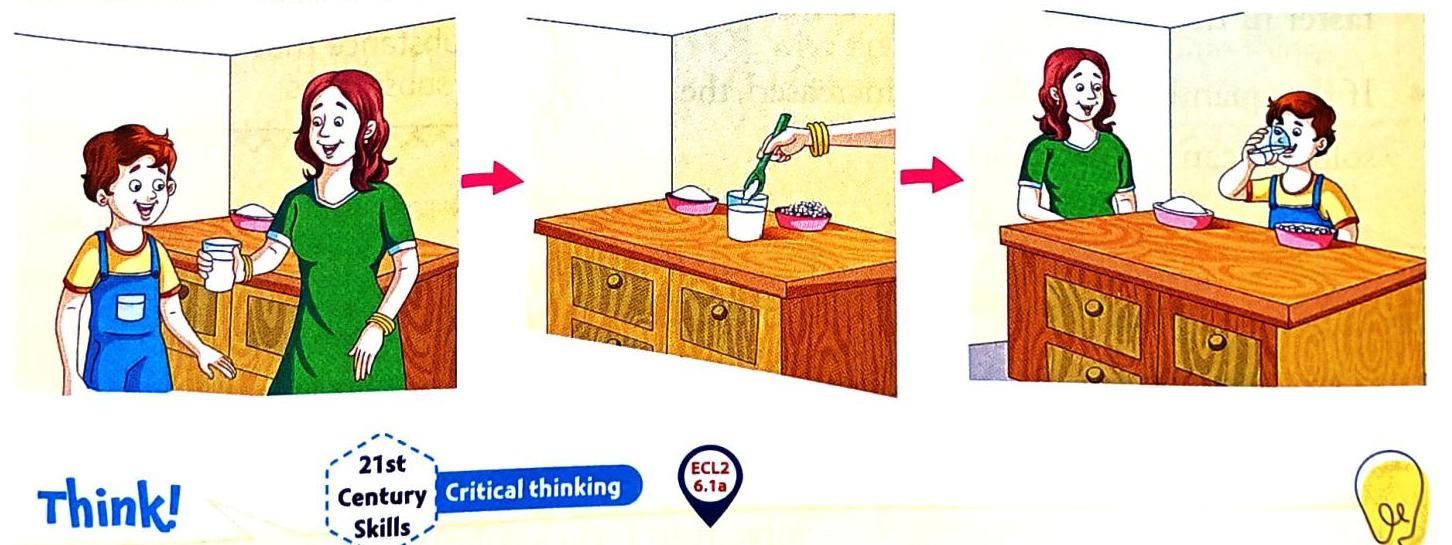
Imagine Aman’s mother making lemonade. When she adds sugar to water, something interesting happens!
Let’s understand the science behind this:
Solution: A solution is a special kind of mixture where one substance completely dissolves into another at a certain temperature. Solute: This is the substance that dissolves. In the lemonade example, the sugar was the solute. When you add salt to water, salt is the solute. Solvent: This is the substance in which the solute dissolves. In the lemonade example, water was the solvent. Water is also the solvent when salt is added to it. Formation: The solute and the solvent together create the solution. For instance, when salt dissolves in water, the result is salty water, which is the solution. Visibility: Once substances like sugar or salt dissolve completely in water, they become invisible. Conditions for Making a Solution
Some factors can make a solution form faster:
Heating the Solvent: If the solvent (like water) is heated, the solute will dissolve more quickly. Stirring the Solute: Stirring the solute into the solvent helps it dissolve faster. Form of the Solute: If the solute is in powdered form, or broken into smaller pieces or particles, it dissolves much faster than larger pieces (like how powdered sugar dissolves faster than sugar granules). Quantity of Solvent: Increasing the amount of solvent can also help a solution form faster. INFO HUB: The Universal Solvent
Water as Universal Solvent: Water is often called the “universal solvent.” Meaning: A universal solvent is a substance that has the ability to dissolve most other substances. This is why water is so important for many processes in nature and in our bodies!
SOLUBLE AND INSOLUBLE SUBSTANCES
Not all substances dissolve in water. This leads us to two important terms:
Soluble Substances: These are substances that do dissolve in a solvent (like water) to form a solution. Example: Sugar and salt are soluble in water. Insoluble Substances: These are substances that do not dissolve in a solvent (like water) to form a solution. Example: Oil and stone are insoluble in water.
OBJECTS THAT FLOAT OR SINK IN WATER
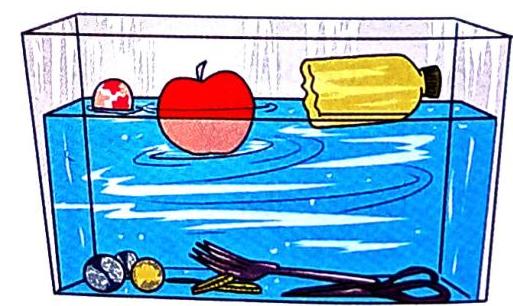
Have you ever wondered why some objects float on water while others sink to the bottom? It’s not just about how big or small an object is! A tiny coin can sink, while a large log can float.
INFO-HUB: Archimedes’ Principle
Key Scientist: It was Archimedes, an ancient Greek scientist, who first explained why things float or sink. The Principle: The secret lies in how much water an object pushes away, or “displaces,” compared to its own weight. Why Objects Sink
Condition: An object will sink if it displaces less water than its own weight. Result: Sinking objects go to the bottom of the water. Why Objects Float
Condition: An object will float if it displaces more water than its own weight. Result: Floating objects stay on the top surface of the water.
Objects sinking and floating in water
Chapter Summary
Let’s review the main ideas we’ve learned about water and its properties.
Water’s Forms: Water exists in three states: Solid: Ice (fixed shape and size) Liquid: Water (no fixed shape or size, takes container shape) Gas: Water vapour (no fixed shape or size, fills available space) Changes in Form: Water can change from one form to another: Melting: Solid (ice) to liquid (water) on heating. Evaporation: Liquid (water) to gas (water vapour) on heating. Condensation: Gas (water vapour) to liquid (water) on cooling. Solidification/Freezing: Liquid (water) to solid (ice) on cooling. Freezing is the reverse of melting. Sublimation: Solid to gas directly. Deposition: Gas to solid directly. A solution is formed when a solute (substance that dissolves) mixes completely with a solvent (substance that dissolves the solute). Water is known as the universal solvent because it can dissolve most substances. Conditions for faster solution: Heating the solvent, stirring, using powdered solute, or increasing solvent quantity. Soluble substances: Dissolve in water (e.g., sugar, salt). Insoluble substances: Do not dissolve in water (e.g., oil, stone). Whether an object sinks or floats depends on the amount of water it displaces compared to its own weight (Archimedes’ Principle). An object sinks if it displaces less water than its own weight. An object floats if it displaces more water than its own weight.
 Self Study
Self Study
Ice cubes (solid)
Ice cubes (solid)
Water (liquid)
Water (liquid)

Water vapour (gas)
Water vapour (gas)





Objects sinking and floating in water
Objects sinking and floating in water


