Skip to content
Call for Central MonitoringRemote Patient Visits + Plan for Out of Window Visits Changes will not be considered a Protocol Violations long as the changed process id documented in NTF and submitted to regulatory
Site qualification, selection, and initiation may be done remotelySome trials closed to enrollment or delaying enrolling patients (33% holding according to survey)Patients already in screening can be rescreened in the futureCreate a memo or policy for risk management and infection controlRemote monitoring is being implementedManage Risk of Investigational Product Supply IssuesIncrease manufacturing and supplyEnsure availability of rescue medications, comparators, and background therapiesFor patients that can self-administer drugs, consider a plan to ship IP directly to patients
Help colleagues cope with new environmentFor remote workers we may need to allow for Job share to manage children at home. One parent works and one manage children and switch.
How to communicate to team when the message can change frequentlyCommunicate status of current processesUpdate as things progress
Be *proactive* in making decisionsDo not just discuss - meetings should result in decision and action planPrioritize ongoing studies above initiating new workCommunication
Sponsor and representatives are granted access to the Source data remotelyVisits can be conducted remotelyUse Home Health Nurse for home visitsPersonal Protection Equipment PPE is need both for staff, patients and testing facilityHave a remote site contact for regulatory compliance and for Reconsent of patients when protocol is modified for remote monitoring and drug supplySafety is important for both the site and the patients as well as the employee
Share items that you find helpful or that you would like to promote in the dedicated post on Facebook in .

 Resources
Resources
Career Toolkit
LinkedIn
Strengths & Preferences


Working From Home & Career Topics
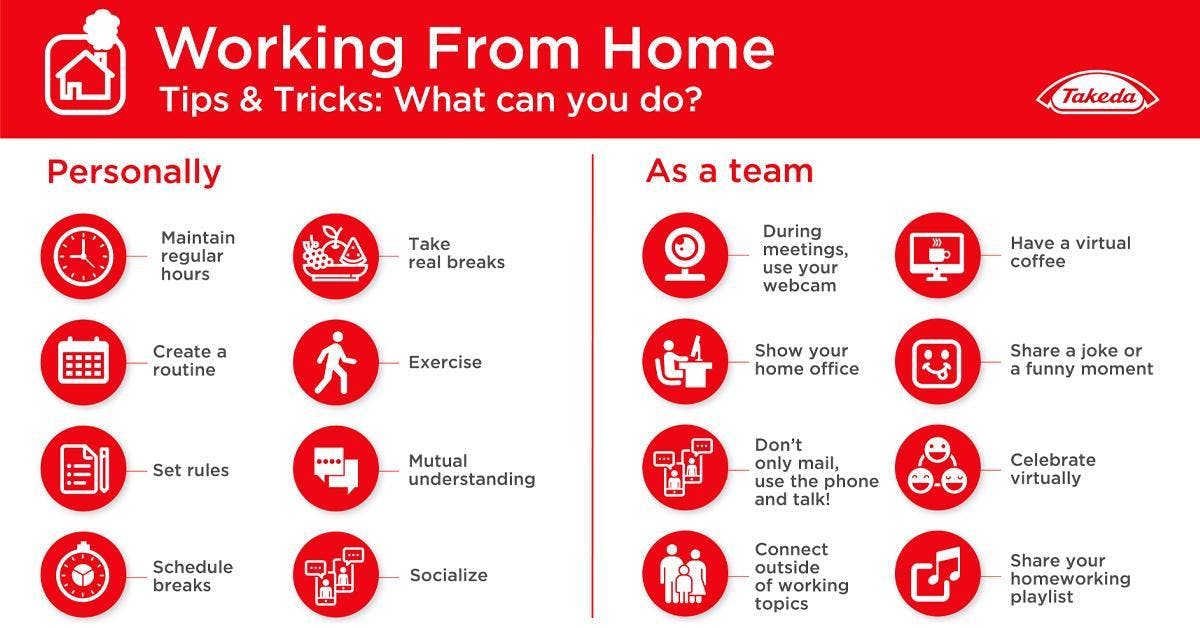
Takeda offers tips for transitioning to the new way of working.

Networking Tips
Career Tips
Watch a rebroadcast of a webinar that discusses how to Reinvent your Reality. Learn how to Stand Out and how to Pivot during any moment of your life with two renowned-authors: Dorie Clark and Jenny Blake
replay the event held 09-April-2020


COVID-19 Resources
FDA Resources
Additional US Government Resources
CTTI Best Practices
DIA DIRECT Webinar Series
session held 08-April-2020
New trials are being put on hold and recruitment of patients is being suspended, given the lack of hospital staff available to manage clinical trials while healthcare systems are gearing up to handle the flood of COVID-19 patients.
Multiple regulatory agencies from across the globe have issued guidance on the management of clinical trials during this COVID-19 pandemic. In order to exchange ideas and provide insights into this impacted world of clinical trials, the DIA Clinical Research Community has brought together a multidisciplinary team of panelists who will provide perspectives from sponsors and clinical trial sites, and on clinical site monitoring, and clinical trial logistics. Each panelist will describe the biggest challenges they are facing today, how they are handling them, what their biggest concerns are, and how they envision clinical trials post-COVID-19.
Moderator –Ghasalek Gouya MD
Site – Faith Holmes MD
Dawn Sauro – Clinical Operations
Alexander Felkovsky – CTS Logistics
Vladimir Misik PHD – Moderator
Leanne – EU Clinical Operations
USFDA/EMA have both issued guidances
Business Impacts to Study Sponsors
Resource Management
Implement a Business Continuity Plan BCP
Leadership Best Practices
Strategies for Clinical Sites
Add Other Resources
Want to print your doc?
This is not the way.
This is not the way.
Try clicking the ··· in the right corner or using a keyboard shortcut (
CtrlP
) instead.