Skip to content
Backbone is unchanged, functional groups that reactHas four valence (outer shell) electrons (covalent)Can bond with 4 other atomsCreate wide array of moleculesAtom unstable unless valence shell is full, reacts with other atoms
Branch of chem that studies carbon compounds
Central part of an organic molecule, determines structure (and function/properties) of moleculeRings, linear, branching, and double bonds Shape determines function -- variety of different molecules
Long chains containing carbon and hydrogenMake up lipidsContains lots of energy (lots of bonds, for ex. fat) Nonpolar (Hydrophobic) - only polar if distribution of charge with an electronegative charge(No O, Su, N -- electronegative atoms)
Structure leads to functionCompounds with same formula but different arrangement of atomsStructuralCarbon backbone is differentGeometricGroups branching off backbone are different (functional groups)Carbons are double bondedFunctional groups on opposite sides = transFunctional groups on same side = cisEnantiomers/StereoisomersMirror images of structureExample: L-Dopa and D-Dopa - only difference is structureL-Dopa treats Parkinson’s, D-Dopa inactive
Participates in chemical reactions***HydroxylAlcohols (OH-)CarbonylKetones (acetone) (carbon double bonded with oxygen)Aldehydes (propanol)CarboxylCarboxylic acids, ie. acetic acidCarbon double bonded to oxygen oxygen single bonded to another oxygenCan be ionized or nonionizedDonate H+ ions to solution, acidicAminoAmines (glycine) Neutral by itselfNH2 - can Ionize and become NH3+, accepts H+ ions (base)SulfhydrylThiols (ethanethiol)SH-PhosphateOrganic phosphates i.e. glyceral phosphate (PO4)Phosphorus bonded to four oxygens two oxygens have negative chargesIn DNA, ATP, GTP etc.Important phosphate groups
Large molecule consisting of many identical/similar building blocks (monomers)
Building blocks for lifeVolcanoes on earth released gasses like Carbon oxides, water vapor, methane, etc.Water formed primordial seas, location of first macromolecules and first living organisms - 3.6 billion years agoStromatolites
Included gasses, rain, and electricityGases reacted with one anotherWater condensed and formed “sea” found monomers, the first macromoleculesAbiotic synthesis of monomers → chemical reactions
Have right elements - CHNOPSElements come together to form the first monomers (amino acids, fatty acids, glycerol, monosaccharides, etc. building blocks)Form polymers (proteins, triglycerides, nucleic acids, etc.)Form cells → bacterial life
Molecular homology - things shared in common due to common ancestry
Fused by dehydration synthesis (remove water) and defused using hydrolysis (add water)

 Unit 1: Chemistry of Life
Unit 1: Chemistry of Life
Carbon
Carbon is essential to life
Organic compound == carbon
Inorganic compound != include carbon
Carbon leads to diversity
Determining factors of organic molecules’ properties
Carbon Backbone - gives structure shape, function
Functional Groups - part of molecule that participates in reactions
Tetravalence
Organic Chemistry
Carbon Backbone
Hydrocarbons
Isomers
Functional Groups - branch off Carbon Backbone
Macromolecules
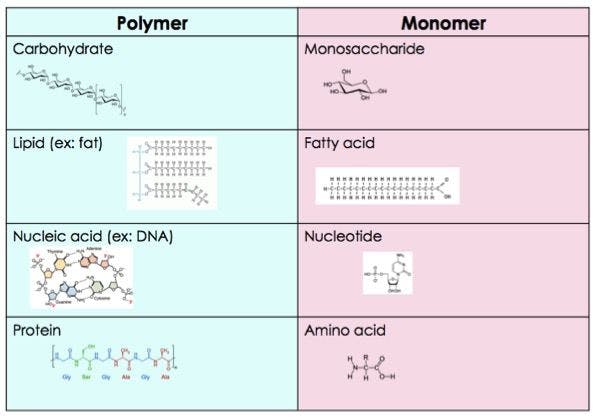

Polymer
Reactions:
First macromolecules
Stanley Miller made experiment simulate conditions on early earth, to create macromolecules abiotically
Earth covered in volcanoes, volatile, releasing gasses in atmosphere → formed primordial seas where sea meets land is where life started.
Start of life - took 1 billion years
DNA (bases) and Proteins (amino acids) compare amounts that organisms share in common, more in common the more similar the organisms are
Monomers are repeating sub units in polymers
Carbohydrates
Lipids
Proteins
Nucleic Acids
Water
Want to print your doc?
This is not the way.
This is not the way.

Try clicking the ··· in the right corner or using a keyboard shortcut (
CtrlP
) instead.