Chapter: 06. Air And Atmosphere
Air And Atmosphere: Your Self-Study Guide
Air is all around us, invisible yet essential for every living thing. Without air, life as we know it would not exist, even for a few short minutes. It’s found over land, in water (dissolved), and covers our entire planet. Though we cannot see, smell, or taste it, we can definitely feel it when it moves—this moving air is called wind.
ATMOSPHERE
The Earth is enveloped by a thin layer of air, known as the atmosphere. This blanket of air extends for many kilometers above the Earth’s surface. The Earth’s gravitational pull keeps this layer of air bound to its surface.
Fig 6.1 Gravity binds the atmosphere. The atmosphere plays a crucial role in maintaining life on Earth:
Temperature Regulation: It helps maintain a stable temperature on Earth. Without the atmosphere, the Earth would experience extreme temperature fluctuations, either burning hot or freezing cold, making life impossible. Protection: It acts as a shield, protecting us from the Sun’s harmful rays.
Demonstrating Air’s Presence
An experiment can clearly show that air is present everywhere, even when a container appears empty.
Take a glass tumbler and a large vessel. Fill the vessel two-thirds with water. Observe the glass tumbler; it appears empty. Invert the glass tumbler and dip it straight down into the water-filled vessel. Tilt the inverted glass tumbler slightly while it is submerged in the water. Observation & Discussion: When the glass is dipped in an inverted position, water does not enter it. This is because the glass is not empty; it is filled with air. When the glass is tilted slightly, air bubbles escape from its mouth, and water then enters the glass. This confirms that the glass was initially filled with air, which escaped to make room for the water.
CHARACTERISTICS OF AIR
Air possesses several distinct characteristics:
It can be compressed easily. It exerts pressure equally in all directions. It is a mixture of gases.
COMPOSITION OF AIR
Before the 18th century, people believed air was a single substance. However, in 1774, a French chemist named Lavoisier demonstrated that air is actually a mixture of many gases.
Fig. 6.2 Composition of air Air’s composition is not fixed but varies slightly depending on the location and time. For example, during the rainy season, air contains more water vapour than in summer. Similarly, the amount of oxygen is less on mountains compared to plains. This variability is one key piece of evidence showing air is a mixture.
Table 6.1 Composition of Air (Approximate Percentages)
Air Is A Mixture Of Gases
The following evidences confirm that air is a mixture rather than a compound:
Variable Composition: The composition of air changes from place to place and with time (e.g., more water vapour in rainy season, less oxygen on mountains). Compounds, by definition, have a fixed composition. No Energy Change on Mixing: When the components of air mix, there is no change in energy (no heat released or absorbed). During the formation of a compound, energy changes are typically observed. Retention of Individual Properties: The gases in air retain their individual properties (e.g., oxygen still supports burning). In a compound, the components lose their individual properties. No Chemical Formula: Air cannot be represented by a chemical formula. Compounds are always represented by a specific chemical formula. Separable by Physical Methods: The components of air can be separated by simple physical methods (e.g., fractional distillation of liquid air). Components of a compound can only be separated by chemical methods.
Components Of Air
Let’s look at the individual components of air in more detail:
Makes up the largest portion of air (approximately 78% by volume). It is an inactive gas, meaning it does not readily react with other substances. It neither burns itself nor supports burning. The second most abundant component of air (approximately 21% by volume). It is very reactive in nature. Oxygen supports burning (combustion). The fact that many substances burn in air demonstrates the presence of oxygen. Think Beyond: If the percentage of oxygen in the air was 50% instead of 21%, the Earth would be much more flammable, and fires would be much more intense and difficult to control. Present in a very small amount (approximately 0.03% by volume). It is released by living beings during respiration. Plants use carbon dioxide to produce their food through the process of photosynthesis. Air contains tiny amounts (traces) of noble gases such as helium and argon. Water is present in the air in its gaseous form, known as water vapour. The amount of water vapour in the air is called humidity. Dust particles and Other Impurities: These are also present in variable amounts in the air.
Demonstrating the Presence of Oxygen and Nitrogen in Air
This activity helps us understand that air contains oxygen, which supports burning, and nitrogen, which does not.
Materials: A candle, water, a trough, a gas jar, and a gas jar stand. Place the gas jar stand in a trough and fill the trough three-fourths with water. Light the candle and fix it onto the gas jar stand. Immediately cover the burning candle with an inverted gas jar. Observe the burning candle and the water level inside the gas jar.
Fig. 6.3 To show the presence of oxygen and nitrogen in air The candle burns for a short period. After some time, the candle flame goes out. As the candle flame goes out, the water level inside the gas jar rises, and the water level in the trough outside the jar decreases. Burning requires oxygen. The candle initially burns because there is oxygen in the limited amount of air trapped inside the gas jar. When all the oxygen inside the gas jar is consumed, the candle stops burning and blows out. The space previously occupied by the consumed oxygen becomes empty, causing the water to rise and fill that space. The significant portion of air that remains inside the gas jar (which does not support burning) is nitrogen, confirming its major presence in air.
Demonstrating the Presence of Carbon Dioxide in Air
This experiment shows that air contains carbon dioxide by reacting it with limewater.
Materials: A test tube, a rubber cork with two holes, a long bent tube, a short bent tube, and limewater (an aqueous solution of calcium hydroxide). Pour some limewater into a test tube. Fit the rubber cork, the long bent tube (dipping into the limewater), and the short bent tube (above the limewater) into the test tube as shown in the figure. Blow air through the long bent tube using an air pump.
Fig. 6.4 Air contains carbon dioxide Air bubbles appear in the limewater. After some time, the limewater turns milky. The air blown through the pump contains carbon dioxide (chemical formula: CO2). Carbon dioxide reacts with limewater (calcium hydroxide, Ca(OH)2) to form an insoluble white solid substance called calcium carbonate (CaCO3). This calcium carbonate precipitate is what makes the limewater appear milky.
Demonstrating the Presence of Water Vapour in Air
This simple activity demonstrates that air contains invisible water vapour.
Materials: A dry glass and ice cubes. Put some ice cubes into the glass. Observe the outer surface of the glass after a few minutes.
Fig. 6.5 Air contains water vapour. Observation & Discussion: After a short time, you will observe tiny drops of water forming on the outer surface of the glass. This happens because the water vapour present in the surrounding air comes into contact with the very cold outer surface of the glass. The cold surface causes the water vapour to cool down and change back into liquid water droplets through a process called condensation.
Demonstrating the Presence of Dust Particles in Air
This activity allows us to see the tiny dust particles that are always present in the air.
Make a room completely dark by closing all doors, windows, and pulling down curtains. Stick a black chart paper on a window that faces the Sun. Make a small hole in the black chart paper. When a thin beam of sunlight enters the darkened room through the small hole, you will observe tiny, shining dust particles moving randomly in a zigzag way within the beam of light.
Fig. 6.6 Observing dust particles in air through a beam of sunlight
USES OF THE COMPONENTS OF AIR
Air is absolutely essential for living organisms. Without air, life on Earth would cease to exist. Let’s explore the importance of its different components.
Oxygen
Oxygen is the most vital component of air, crucial for two main processes: respiration and combustion (burning).
Respiration
Respiration is a fundamental biochemical process that occurs in all living organisms. During respiration, oxygen reacts with digested food material (glucose) to produce carbon dioxide, water, and essential energy. This energy powers all life activities.
\text {Glucose (digested food)} + \text {Oxygen} \xrightarrow{\text {respiration}} \text {Carbon dioxide} + \text {Water} + \text {Energy}
Without oxygen, body cells cannot use the digested food to produce energy. We obtain oxygen from the air. When we breathe in (inhalation), we take in air rich in oxygen. When we breathe out (exhalation), we release air rich in carbon dioxide. The combined process of inhalation and exhalation is called breathing.
Inhaled air is rich in oxygen. More water vapour than inhaled air. More carbon dioxide than inhaled air. Less oxygen than inhaled air (because oxygen is used by body cells during respiration). Demonstrating More Carbon Dioxide in Exhaled Air
This activity compares the amount of carbon dioxide in fresh air versus exhaled air.
Materials: Two conical flasks (F1 and F2) with equal amounts of limewater, glass tubes, rubber corks, and pinch corks (A and B). Set up the apparatus as shown in the figure, marking the flasks F1 and F2. To test fresh air: Open pinch cork A and close pinch cork B. Suck fresh air through the mouth (M). Observe how quickly the limewater in flask F1 turns milky. To test exhaled air: Close pinch cork A and open pinch cork B. Exhale air through the mouth (M). Observe how quickly the limewater in flask F2 turns milky.
Fig. 6.7 Limewater turns milky rapidly by exhaled air. Observation: The limewater in flask F2 (receiving exhaled air) turns milky much more rapidly than the limewater in flask F1 (receiving fresh air). Discussion: This rapid change indicates that exhaled air contains a significantly higher concentration of carbon dioxide compared to fresh air, which makes the limewater turn milky faster. Oxygen Cylinders
In situations where oxygen in the environment is insufficient, people use oxygen cylinders for breathing:
Mountaineers: Carry oxygen cylinders when climbing high mountains because air at high altitudes contains less oxygen. Divers: Carry oxygen cylinders when diving into the deep sea, as they cannot use the oxygen dissolved in water directly. Hospital Patients: Often receive extra oxygen through cylinders when their bodies need more support for respiration. Oxygen in Soil and Water
Soil Animals: Animals living in the soil (like earthworms) obtain oxygen from the air trapped between soil particles. During heavy rains, soil gets filled with water, forcing these organisms to come out to breathe. Aquatic Life: Aquatic plants and animals utilize the oxygen that is dissolved in water. Demonstrating Air in Soil
This activity shows that air is present within soil.
Materials: A beaker, soil, and water.  Self Study
Self Study

Fig 6.1 Gravity binds the atmosphere.
Fig 6.1 Gravity binds the atmosphere.
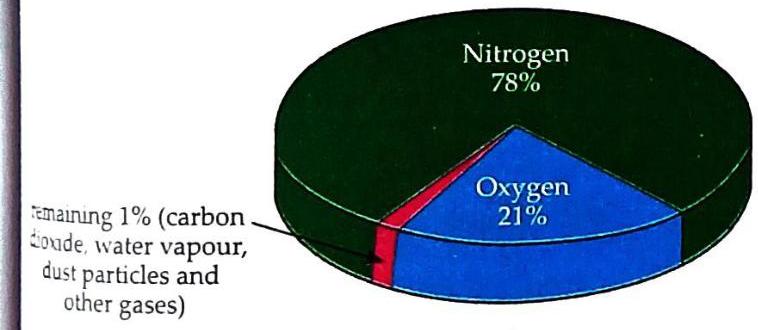
Fig. 6.2 Composition of air
Fig. 6.2 Composition of air
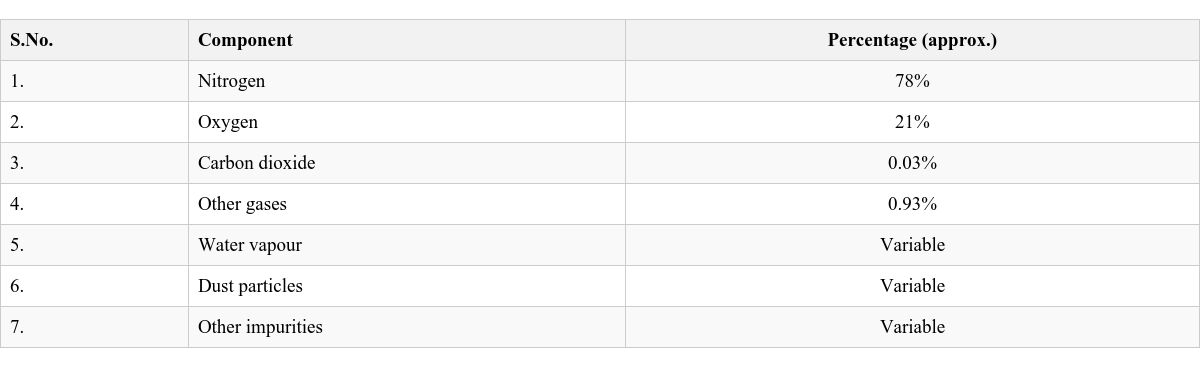

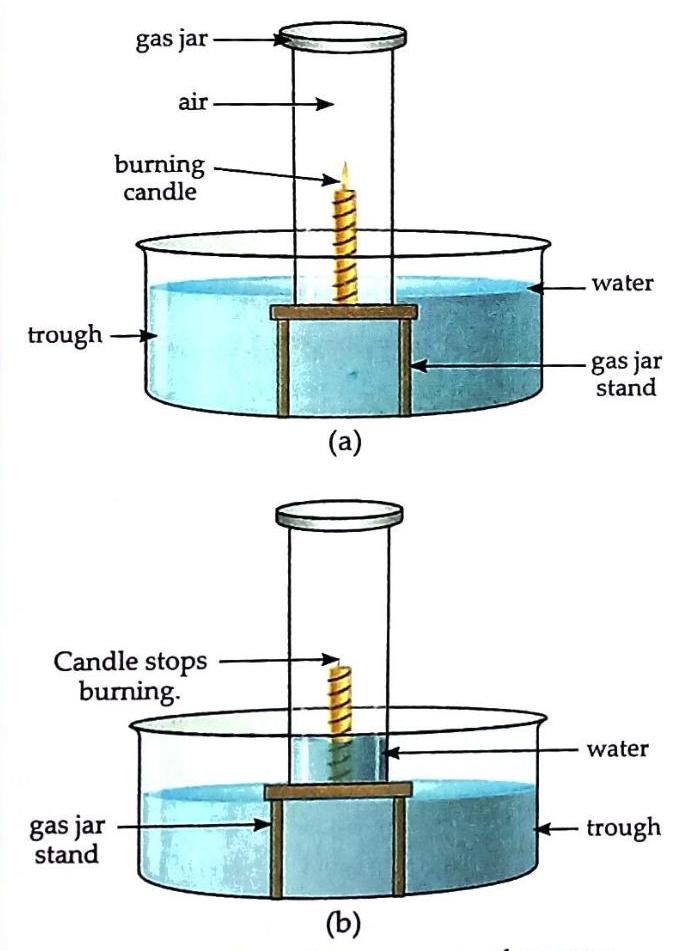
Fig. 6.3 To show the presence of oxygen and nitrogen in air
Fig. 6.3 To show the presence of oxygen and nitrogen in air
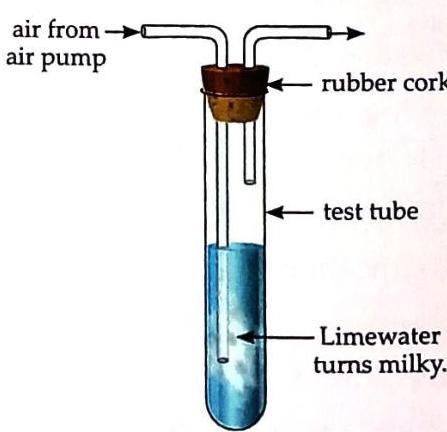
Fig. 6.4 Air contains carbon dioxide
Fig. 6.4 Air contains carbon dioxide
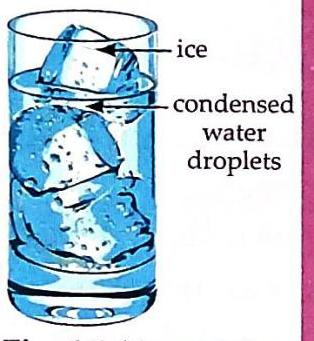
Fig. 6.5 Air contains water vapour.
Fig. 6.5 Air contains water vapour.
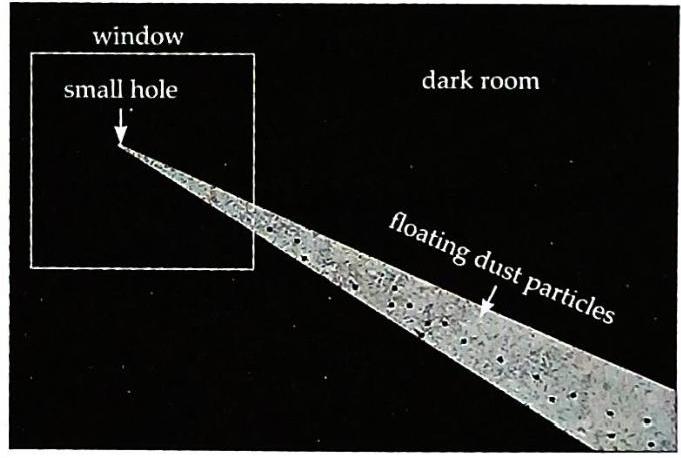
Fig. 6.6 Observing dust particles in air through a beam of sunlight
Fig. 6.6 Observing dust particles in air through a beam of sunlight



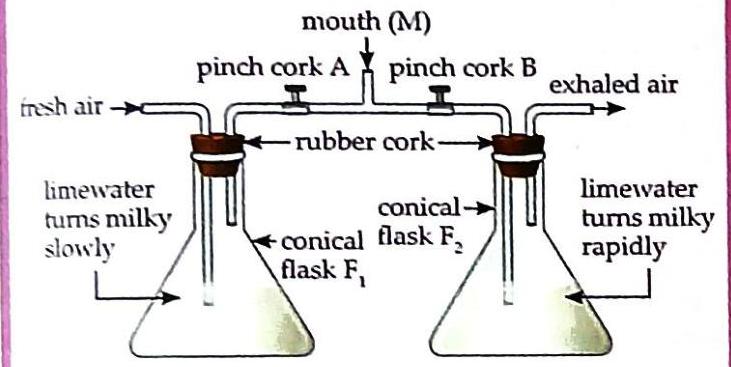
Fig. 6.7 Limewater turns milky rapidly by exhaled air.
Fig. 6.7 Limewater turns milky rapidly by exhaled air.