Chapter: 05. Water
Chapter 5: Water - Your Essential Guide
Water is one of the most vital substances for all life on Earth, supporting the survival of plants, animals, and humans alike.
1. Occurrence of Water
Water is abundant on Earth, covering two-thirds of its surface.
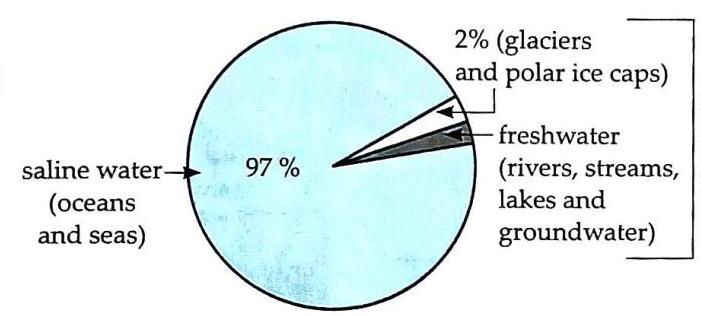
Saline Water: About 97% of Earth’s total water is found in seas and oceans. Frozen Water: 2% is present as glaciers and polar ice caps. Freshwater: Only 1% is available in rivers, ponds, lakes, and as underground water.
Fig. 5.1 Percentage of different forms of water on earth Presence in Atmosphere: Water exists in the atmosphere as vapour, mist, and clouds. Presence in Living Organisms: All living organisms contain water. It is present in fruits, vegetables, and even in substances that appear dry. About 70% of the human body is water. Conclusion: Water is everywhere, and life without it is unimaginable.
2. Water - A Compound
Water is not an element but a compound.
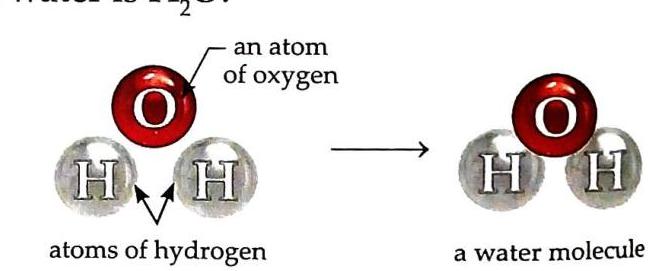
Nature: Transparent liquid. Discovery: Proved to be a compound by Henry Cavendish in 1781. Composition: Made up of two elements: hydrogen and oxygen. One molecule of water (H₂O) contains two atoms of hydrogen and one atom of oxygen. The proportion of hydrogen and oxygen in water is fixed at 2:1.
Fig. 5.2 Water is a compound of hydrogen and oxygen. Chemical Formula: H₂O.
3. Properties of Water
Water possesses distinct physical and chemical properties.
Pure Water Characteristics: Boiling Point: 100°C (changes from liquid to gas). Freezing Point: 0°C (changes from liquid to solid). States of Existence: Water exists in three states: Universal Solvent: Water is known as a universal solvent because a large number of substances can dissolve in it.
4. Sources of Water
The main sources of water on Earth are rainwater, surface water, and underground water.
4.1. Rainwater
Source: Directly from rain. Contents: Contains dissolved gases like oxygen and carbon dioxide. Purity: Considered the purest form of water available on Earth. Impurities: Sometimes may contain dust. 4.2. Surface Water
Water found on the surface of the Earth is called surface water.
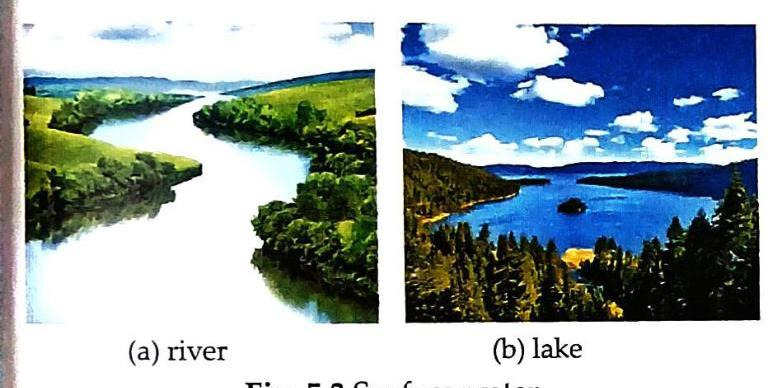
Examples: Water in rivers and lakes. Source: Comes from rain and melting snow on mountains. Flow: Water flows down mountains into rivers. Impurities: Carries various impurities due to flow: Suspended impurities (sand, clay particles, organic matter) Harmful microorganisms (bacteria) Usability: Cannot be directly used for domestic or industrial purposes without treatment.
Fig. 5.3 (a) river, (b) lake. Largest Source: Oceans and seas hold the largest amount of water on Earth. Contents: Contain a large amount of dissolved impurities, primarily common salt. Salinity: Sea water is saline due to high common salt concentration. Impurity Accumulation: All rivers and streams eventually flow into the sea, bringing their impurities with them. Usability: Not suitable for drinking or irrigation. Desalination: The process of removing dissolved salts from sea/ocean water to make it usable. Activity 1: Demonstrating Salt in Seawater Aim: To show that sea water contains salt. Equal amounts of rainwater and sea water are placed in separate china dishes. Heated on a tripod stand until all water evaporates. Rainwater dish: No residue. Sea water dish: Large amount of salt residue. Conclusion: Sea water contains dissolved salts, unlike rainwater. Activity 2: Saline Water and Plant Growth Aim: To show that saline water is not suitable for plants. Two healthy potted plants are taken. One is watered with tap water, the other with saline water regularly. Plant watered with tap water: Grows well. Plant watered with saline water: Does not grow well. Conclusion: Saline water inhibits plant growth, making it unsuitable for irrigation. 4.3. Underground Water
Water that seeps through the soil and collects below the surface.
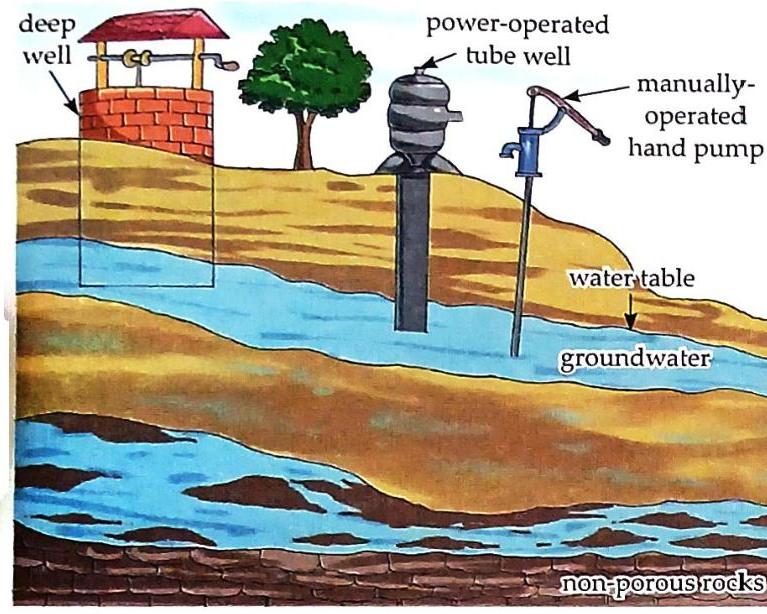
Formation: Rainwater seeps through the soil and collects on a layer of hard, non-porous rocks. Reservoir: This collected water below the surface is called underground water. Water Table: The upper limit of the underground water reservoir is known as the water table.
Fig. 5.4 Groundwater can be pumped out by a well, tube well or a hand pump. Extraction: Obtained by digging deep wells, tube wells, and using hand pumps. Spring Water: At some places, underground water emerges from the ground naturally in the form of springs.
Fig. 5.5 Groundwater coming out in the form of spring Contains dissolved impurities. Free from suspended impurities because water gets filtered through different soil layers. Taste Variation: The taste of underground water varies from place to place due to different soil compositions. Mineral Properties: Water from some wells and springs contains minerals that may have medicinal properties.
5. States of Water
Water can exist in three different physical states.
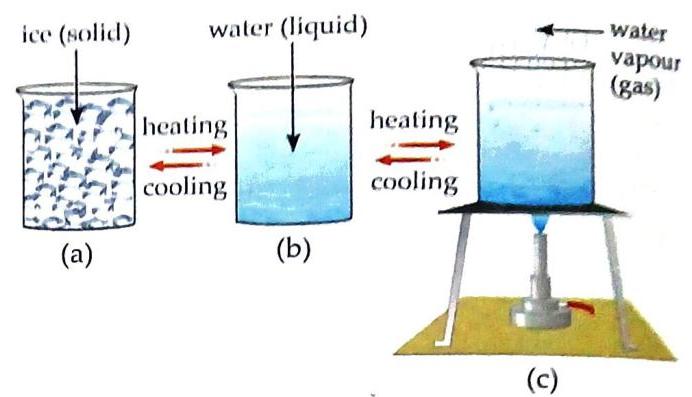
Gaseous State: Water vapour or steam
Fig. 5.6 States of water 5.1. Different States of Water in Nature
Water naturally occurs in all three states.
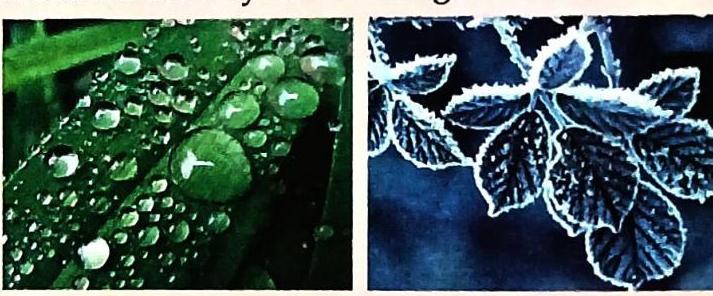
Solid State in Nature: Found in glaciers and on mountains as snow. Liquid State in Nature: Found in oceans, seas, rivers, lakes, ponds, and as rainwater. Gaseous State in Nature: Present as water vapour in air. Dew: Tiny droplets of water formed by the condensation of water vapour in the air on cold objects (e.g., leaves, grass). Frost: Formed when dew freezes. Fog: Tiny droplets of water formed by water vapour near the ground. Mist: Suspended tiny droplets of water in the air.
Dew & Frost
Fog & Mist 5.2. Interconversion of States of Water
Water can change from one state to another based on temperature.
Normal Condition: Water exists in the liquid state. Liquid to Gas: When water is heated to 100°C, it changes into water vapour (steam). Solid to Liquid: When ice (solid) is heated, it changes into water (liquid). Gas to Liquid: When water vapour or steam is cooled, it changes back into water. Liquid to Solid: When water is cooled to 0°C, it changes into ice (solid). Natural Interconversion: The interconversion of water states also occurs continuously in nature as the water cycle.
Fig. 5.7 Different states of water 5.3. Water Cycle
The continuous movement of water from the Earth’s surface to the atmosphere and back.
Steps of the Water Cycle: Evaporation: Heat from the Sun causes water from seas, oceans, rivers, lakes, and other water bodies to evaporate. Plants also release water vapour through transpiration. Water vapour rises into the atmosphere. Cooling and Condensation: As water vapour rises, it cools (temperature decreases with height). At a certain height, it condenses to form tiny water droplets, which float with dust particles to form clouds. Precipitation: Tiny water droplets in clouds join to form larger, heavier droplets. When they become too heavy for air to hold, they fall as rain. In very low temperatures, water droplets freeze and fall as hail or snow. Some rainwater seeps into the soil to become groundwater. Groundwater is extracted for various purposes and eventually reaches rivers and oceans. Some rainwater freezes as ice on mountains, which then melts and flows into rivers and oceans. Repetition: This entire cycle of evaporation, condensation, and precipitation repeats continuously. Definition: The continuous movement of water from the Earth’s surface as water vapour to the atmosphere and from the atmosphere back to the Earth’s surface as rain, hail, or snow. Importance of Water Cycle: Maintains Water Balance: Restores water lost from the Earth’s surface, maintaining water levels on land. Controls Climatic Conditions: Plays a crucial role in regulating global climate.
6. Anomalous Behaviour of Water
Unlike most liquids, water exhibits unusual expansion when cooled below a certain temperature.
Heating: Expands, volume increases, density decreases. Cooling: Contracts, volume decreases, density increases. When cooled, water contracts normally only until 4°C (volume decreases, density increases). Below 4°C: Water starts expanding. Its volume increases, and its density decreases until it reaches 0°C. Freezing: At 0°C, water freezes into ice. Result: Ice is lighter than water, which is why it floats on water. Significance for Aquatic Life: This anomalous expansion is crucial for the survival of aquatic plants and animals in colder regions. During winter, when air temperature falls below 0°C, water in ponds, lakes, and rivers freezes on the surface. Ice as Insulator: Ice is a poor conductor of heat. It forms an insulating layer on the surface, preventing heat flow from the water below to the colder atmosphere. Maintaining Water Temperature: This insulation keeps the deeper layers of water bodies at a temperature of 4°C. Survival: This allows aquatic life to survive in the liquid water beneath the ice.
Fig. 5.8 A frozen lake
7. Importance of Water
Water is fundamental for all forms of life and serves numerous purposes.
Essential for All Living Organisms: Life would not exist without water. Hydration: Drink 8-10 glasses daily. Nutrient Absorption: Dissolves food nutrients for body absorption. Waste Removal: Dissolves waste materials, removed as sweat and urine. Temperature Regulation: Regulates body temperature through sweating (evaporation cools skin). Growth: Helps in seed germination and plant growth. Photosynthesis: Essential for making food. Nutrient Absorption: Dissolves soil nutrients for root absorption. Transport Medium: Transports minerals and nutrients within the plant. Domestic: Cooking, bathing, washing clothes/utensils, firefighting. Industrial: Used in industries (paper, cloth, medicine) that require large water quantities. Electricity Generation: Used in hydroelectric power plants to produce electricity. Transportation: Oceans, seas, and large rivers serve as transport mediums for people and goods (boats, ships). Cooling Agent: Used in vehicle radiators to cool engines. Recreational Sports: Medium for swimming, boating, water skiing. Heating: Used in cold climates to keep houses warm.
8. Potable Water
Water that is fit and safe for drinking purposes.
Definition: Water fit and safe for drinking. Daily Intake: Should drink 8-10 glasses every day. Appearance: Colourless, transparent, and odourless. Germ-Free: Should be free from disease-causing microorganisms. Impurity-Free: Should be free from suspended impurities. Mineral Content: Should contain a small amount of essential minerals and salts (magnesium, calcium, sodium), which are necessary for body growth and development.
9. Water-Borne Diseases
Diseases caused by consuming contaminated water containing germs.
Cause: Germs (disease-causing microorganisms like bacteria and viruses) present in unclean water from various sources. Definition: Diseases caused by drinking contaminated water. Examples of Water-Borne Diseases: Diarrhoea: Frequent discharge of loose watery stool, leading to loss of water and minerals from the body. Gastroenteritis and Cholera: Acute diarrhoea accompanied by frequent vomiting. Caused by toxins from viruses, bacteria, and parasites leading to inflammation. Dehydration: Excessive loss of water from the body due to untreated diarrhoea, gastroenteritis, or cholera. Dehydration can be fatal. Jaundice: Affects the liver. Typhoid: Affects the intestine and causes high fever. Necessity: We need to drink purified water to prevent these diseases.
10. Purification of Water
Water purification involves removing impurities and killing germs to make it safe for use.
Goal: To kill germs and remove impurities from water. 10.1. Sterilisation (Germ Removal)
The process of removing germs from water.
Boiling: Boiling water for about 20-30 minutes can destroy harmful microorganisms. Exposure to Air and Sunlight: Germs can be killed by blowing air under pressure through filtered water. Chemical Treatment (Chlorination): Bleaching powder, rich in chlorine, is used to kill germs in water. 10.2. Large Scale Purification of Water (Water Treatment Plant)
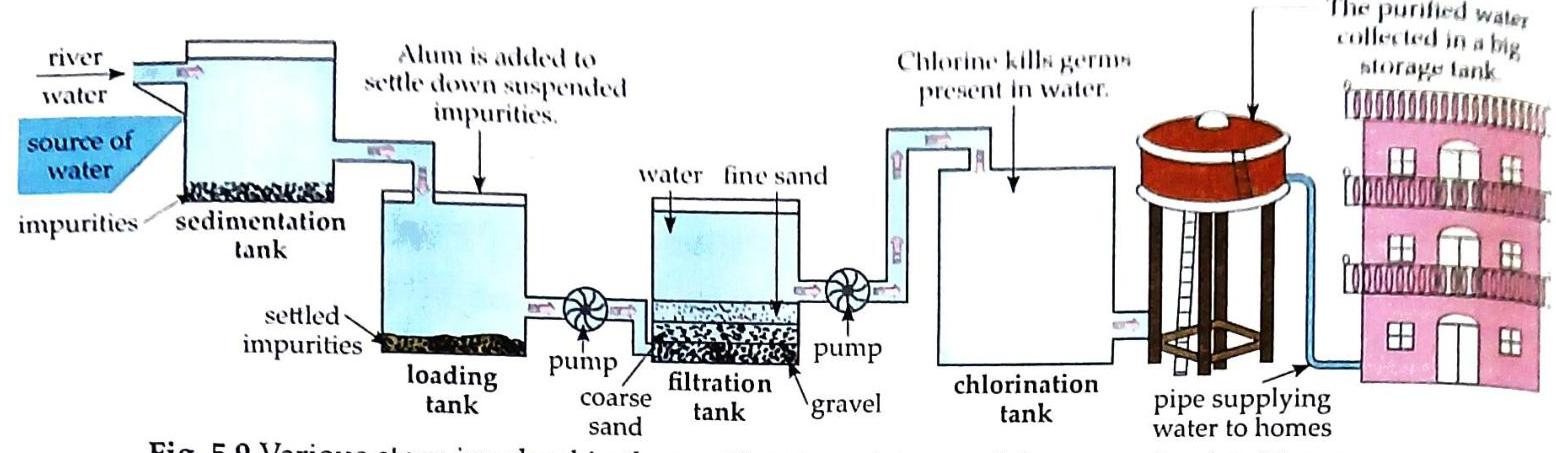
Water from rivers or lakes is purified in a water treatment plant before it reaches homes.
Water is allowed to stand for a day. Large insoluble impurities settle at the bottom. Light insoluble impurities remain suspended. Water from sedimentation tank is passed here. Alum is added to settle suspended impurities faster (coagulation). Water from loading tank is passed through layers of sand, gravel, and charcoal. These layers filter out the remaining lighter suspended impurities. Water from filtration tank receives bleaching powder (rich in chlorine). Chlorine kills all germs present in the water, making it safe for drinking. After chlorination, purified drinking water is stored in large tanks. From these tanks, it is supplied to homes through pipes.
Fig. 5.9 Various steps involved in the purification of river or lake water for drinking purpose 10.3. Purification of Water at Home
Methods to purify tap water for drinking and cooking.
Water can be filtered through a fine muslin cloth to remove suspended impurities. Modern water purifiers often use Reverse Osmosis (RO) technique. Activity 3: Tap Water Contains Dissolved Impurities Aim: To show that tap water contains some dissolved impurities. Method: Tap water is heated in a watch glass over a beaker of boiling water until it evaporates. Observation: Concentric rings of solid substances (impurities) are observed on the watch glass. Conclusion: Tap water contains dissolved impurities. Boiling water for 10-15 minutes kills germs. It is the simplest and easiest method for small-scale water purification.
Fig. 5.10 Boiling water Treating with Certain Chemicals: Adding a small quantity of bleaching powder to filtered water kills germs by releasing chlorine. Chlorine tablets (e.g., one tablet for 25 litres of water) and potassium permanganate are also used to make water germ-free. Exposure to Ultraviolet (UV) Radiation: Exposing water to UV radiation for some time makes it germ-free. Many modern water filters use UV radiation to kill germs.
11. Distilled Water
The purest form of water, obtained through distillation.
Definition: The purest form of water, containing no salt or mineral. Obtaining Distilled Water: Achieved by the process of distillation.  Self Study
Self Study