Chapter: 04. Mixtures Separations Of Mixtures
Chapter 4: Mixtures — Separation Of Mixtures
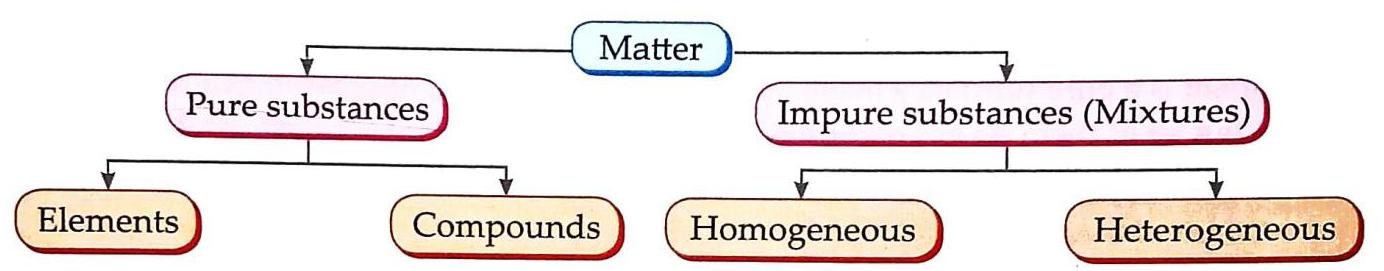
In the previous chapter, we learned that all substances can be divided into two groups: pure substances and impure substances (mixtures) (Fig. 4.1).
Fig. 4.1 Classification of matter
Pure Substance
A pure substance is a substance that contains the same kind of particles (atoms or molecules).
They are either elements or compounds. They possess a definite set of physical and chemical properties. Silver: An element, containing the same kind of atoms. Sugar: A compound, containing the same kind of molecules.
Impure Substance
An impure substance is a substance that contains two or more pure substances (elements or compounds or both) physically mixed in any proportion.
A mixture is an impure substance.
Mixture
A mixture consists of two or more pure substances (elements or compounds) mixed together in any proportion, that do not undergo any chemical change and retain their individual properties.
Air: Consists of oxygen, nitrogen, carbon dioxide, water vapour, and dust particles. Molecules of components are different. Properties Of A Mixture
Composition: It consists of two or more substances (elements or compounds or both) mixed together physically. Fixed Composition: It does not have any fixed composition. The components of a mixture are present in different proportions. Example: The amount of oxygen in the air at high altitudes is much less than in plains, showing variable composition. Retention of Properties: The components of a mixture retain their individual properties. Thus, it shows the properties of its components. Example: In air, oxygen supports burning, and carbon dioxide is used for extinguishing fire, retaining their original properties. Definite Property: It does not have any definite property. The properties can be changed by changing the quantity of components. Example: A mixture of sugar and water does not have fixed properties. Changing the quantity of sugar or water changes the sweetness. (Note: This does not change their individual properties). Self-Study Insight (from Activity 1): Aim: To show that a mixture does not have definite properties. Method: Three glasses with water, adding 1, 2, and 3 teaspoons of sugar respectively. Stir and taste. Fig. 4.2 Glasses having different quantities of sugar Observation: Water in all glasses is sweet, but sweetness varies (glass with 3 spoons sugar is sweetest). Discussion: All glasses contain a sugar-water mixture, but properties (sweetness) are different due to varying amounts of constituents (sugar). Melting and Boiling Points: It does not have fixed melting and boiling points. These points depend on the proportions of components. Separation of Components: The components of a mixture can be separated by simple physical methods because they do not combine chemically. Energy Change: Generally, energy is neither released nor absorbed during the formation of a mixture.
Types Of Mixtures
Mixtures are broadly classified into two types: homogeneous and heterogeneous.
1. Homogeneous Mixture
A mixture in which its components are mixed uniformly in such a way that they cannot be seen separately is called a homogeneous mixture.
Salt solution (salt and water particles cannot be seen separately). 2. Heterogeneous Mixture
A mixture in which its components are not mixed uniformly and can be seen separately is called a heterogeneous mixture.
Soil (contains sand, clay, minerals; composition varies). Sulphur and iron filings
Formation Of A Mixture
Different types of mixtures are formed by mixing solid, liquid, and gaseous substances. These substances are mixed in various proportions based on their properties and uses. Depending on the physical states of its components, a mixture can exist in solid, liquid, or gaseous forms.
Examples of Mixtures and their Components: Milk: Mixture of fats, carbohydrates, proteins, vitamins, and water in different proportions. Honey: Mixture of sugar, water, and other carbohydrates. Fruit juice: Mixture of water, sugar, and fruit pulp. Tap water: Mixture of water and some dissolved salts. Brass: Mixture of copper and zinc. Bronze: Mixture of copper and tin. Self-Study Insight (from Activity 2):
Aim: To study the mixture of oil and water. Add mustard oil drops to water in a glass bottle. Observe. Close bottle, shake vigorously. Observe. Allow mixture to stand. Observe again. Observation and Discussion: Oil forms a layer on water because it is lighter than water.
Fig. 4.3 Oil in water On shaking, layers mix to form a milky mixture called an emulsion. Emulsion: A mixture of two or more liquid components that are immiscible (cannot be mixed completely). When left to stand, oil and water separate again.
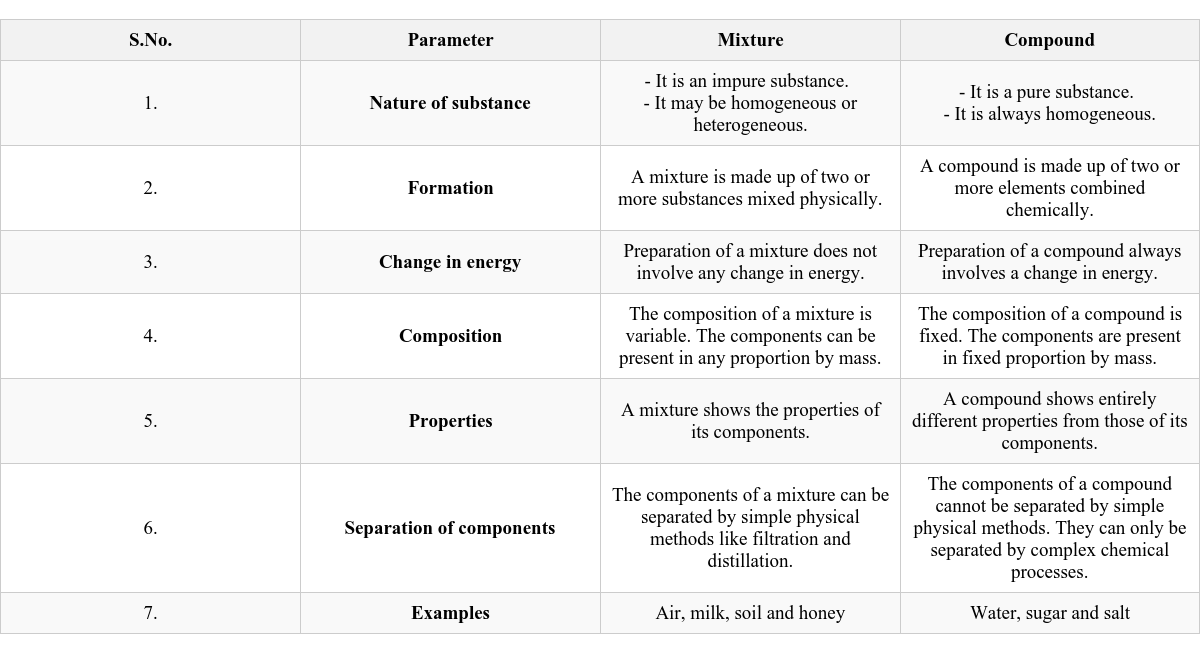
Differences Between A Mixture And Compound
Let’s understand the differences by exploring an experiment and then a comparison table.
Self-Study Insight (from Activity 3):
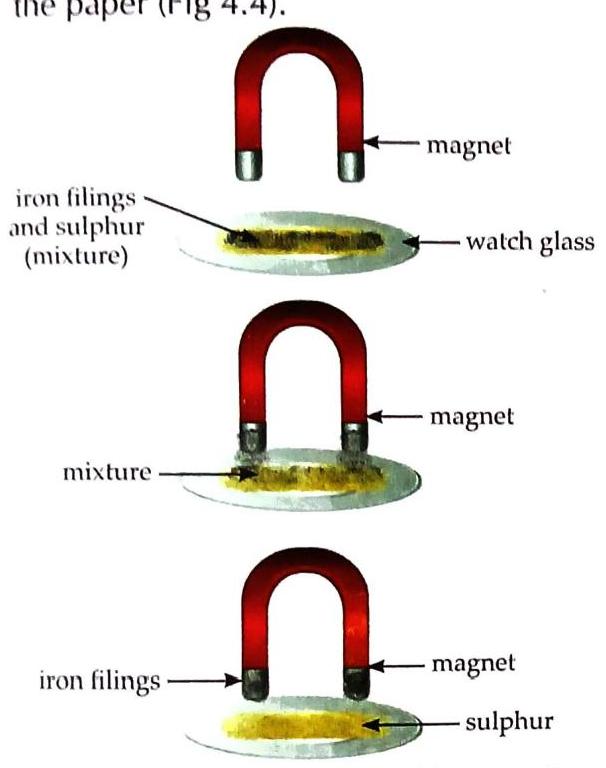
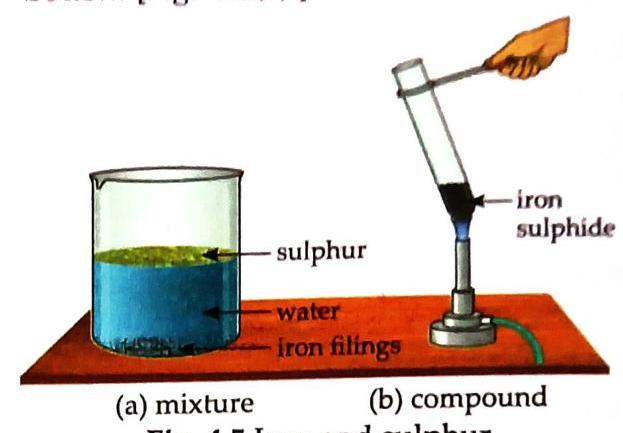
Aim: To show the differences between a mixture and a compound (using iron filings and sulphur). Grind iron filings and sulphur to a powder. Observe powdered mixture under magnifying glass. Bring a magnet over the mixture. Observe. Put some mixture in water, stir, let stand. Observe. Heat a pinch of mixture in a test tube. Observe the resulting substance. Observe the dark grey substance (after heating) under magnifying glass. Bring a magnet over the dark grey substance. Put some dark grey substance in water, stir, let stand. Observe. Observation and Discussion: Powdered Mixture (Mixture): Under magnifying glass: Yellow sulphur particles and greyish-black iron particles are visible separately. With magnet: Iron filings cling to the magnet, leaving sulphur behind (Fig 4.4). Fig. 4.4 Separation of iron filings and sulphur from their mixture In water: Sulphur rises up, iron filings sink to the bottom [Fig. 4.5(a)]. Heated Substance (Compound - Iron Sulphide): When iron and sulphur mixture is heated, it reacts chemically to form a dark grey substance (iron sulphide) [Fig. 4.5(b)]. Fig. 4.5 Iron and sulphur Under magnifying glass: Sulphur and iron particles are not seen separately. With magnet: The dark grey substance (iron sulphide) does not cling to the magnet. In water: Neither sulphur floats nor iron filings sink. Conclusion: Iron and sulphur retain their properties in the mixture but form a new substance with entirely different properties (iron sulphide) when chemically combined. Table 4.1 Differences between Mixture and Compound
Why Air Is A Mixture And Water A Compound
Air and water are essential for life. Let’s compare their properties to understand why air is a mixture and water is a compound.
Table 4.2 Differences between Air and Water
Need For Separation Of Components Of A Mixture
We need to separate the components of a mixture for the following reasons:
To remove undesirable and harmful substances: Example: Removing small stones and husk from rice and pulses. Removing impurities from drinking water. To get useful components: Example: Separating common salt from sea water for food and nutrition. Obtaining petrol, diesel, kerosene, and LPG from petroleum. To get a pure sample of a substance: Example: Separating dissolved salts from tap water to obtain pure water (distilled water).
Methods Of Separation
The process of segregating the components of a mixture to get a pure substance is called separation.
There are different methods of separating the components of a mixture, depending upon:
The properties of the components. These properties may include: Separation Of Solid-Solid Mixtures
The components of a solid-solid mixture can be separated by hand-picking, winnowing, sieving, magnetic separation, and sublimation.
1. Hand-picking
The method of separating undesirable substances from a mixture by hand is called hand-picking.
The mixture is present in a small quantity. The component to be separated forms a small portion of the mixture. The components are different in size, colour, and shape. Example: Removing small stones from gram and pulses.
2. Winnowing
This method is used for separating light substances from heavier ones. The method of separating heavier and lighter components of a mixture by wind is called winnowing.
A farmer takes a mixture of grains and husk in a winnowing basket.  Self Study
Self Study