Chapter: 03. Elements And Compounds
Elements And Compounds: A Self-Study Guide
In the previous chapter, we learned that everything around us is made up of matter, which can exist as a solid, liquid, or gas based on its physical state. In this chapter, we will explore the chemical classification of matter.
Chemical Classification of Matter
When we compare substances like sugar and soil, we observe distinct differences. Sugar particles are similar in color, shape, and size, indicating it is a pure substance. Soil, however, contains particles of various kinds (like clay, gravel, and dry leaves), making it an impure substance or a mixture.
Pure Substance: A substance that contains only one kind of particles (atoms or molecules). Impure Substance or Mixture: A substance that contains two or more pure substances (elements or compounds or both) physically mixed in any proportion. The classification of matter can be visualized as follows:
Fig. 3.1 Classification of matter
Pure Substance
A pure substance is characterized by containing identical particles, whether they are atoms or molecules.
Properties Of Pure Substances
Definite Chemical Composition: They have a specific arrangement of elements. Uniform Composition: Their composition is consistent throughout. Definite Physical and Chemical Properties: They exhibit unchanging physical and chemical characteristics. Fixed Melting and Boiling Points: They melt and boil at precise temperatures. For example, pure water boils at 100°C and freezes at 0°C. Adding impurities like sugar or salt alters these points. Characteristic Colour, Odour, and Taste: They possess unique sensory attributes. Cannot be Split by Physical Methods: They cannot be broken down into simpler substances using physical processes. Examples: Water is a pure substance, made only of water molecules (hydrogen and oxygen in a 1:8 ratio by mass).
Homogeneous Substances: Substances that have a uniform composition throughout. Heterogeneous Substances: Substances that do not have a uniform composition. Classification Of Pure Substances
Pure substances are broadly categorized into two types:
Elements
All substances on Earth are built from simpler substances called elements. An element is a pure substance made up of only one kind of atoms that cannot be broken down into two or more simpler substances by any physical or chemical method.
For example, iron (Fig. 3.2) is an element composed solely of iron atoms. It cannot be broken down further by physical or chemical means. Similarly, sulphur (Fig. 3.3) is an element.
Fig. 3.2 Iron powder
Fig. 3.3 Sulphur powder Atom: The smallest unit of an element. DO WE KNOW? (Science in Life) (NEP GUIDELINES)
Scientists have identified 118 elements. Out of these, 92 elements are found naturally in rocks, soil, air, and water. The remaining 26 elements have been created artificially. Other examples of elements include oxygen, hydrogen, zinc (Fig. 3.4), nitrogen, carbon, copper, silver, gold, and tin.
Fig. 3.4 Zinc
Classification Of Elements
Elements are categorized into four main groups based on their properties:
1. Metals
Elements like gold, silver, iron, copper, mercury, and aluminium are examples of metals.
Properties of Metals
State at Room Temperature: Generally solid. Exception: Mercury (Fig. 3.5) is a liquid metal.
Fig. 3.5 Mercury-a liquid metal Hardness and Strength: Generally hard and strong. Exception: Sodium (Fig. 3.6) and potassium are soft metals.
Fig. 3.6 Sodium - a soft metal Lustre: Lustrous or shiny. Exception: Sodium is a non-lustrous metal. Malleability: Malleable, meaning they can be hammered into thin sheets or foils (Fig. 3.7). Exception: Zinc is brittle and breaks on hammering.
Fig. 3.7 Hammering of a metal into thin sheet Ductility: Ductile, meaning they can be drawn into thin wires (Fig. 3.8).
Fig. 3.8 Copper wires Conductivity: Good conductors of heat and electricity (Fig. 3.9).
Fig. 3.9 Aluminium is used in transmission cable Melting and Boiling Points: High melting and boiling points. Exception: Sodium and potassium have low melting points. Sonorousness: Sonorous, producing a ringing sound when struck.
2. Non-metals
Elements such as oxygen, hydrogen, nitrogen, carbon, phosphorus, and chlorine are non-metals.
Properties of Non-metals
State at Room Temperature: Either solids (like sulphur and phosphorus) or gases (like oxygen and nitrogen). Exception: Bromine is the only non-metal found in the liquid state. Hardness: Generally soft. Exception: Diamond (a form of carbon) is an extremely hard non-metal. Note: Carbon exists as soft solids (charcoal, coal) and hard solids (diamond, graphite). Lustre: Non-lustrous (not shiny or dull). Exception: Iodine (Fig. 3.10) is lustrous.
Fig. 3.10 Iodine - a lustrous non-metal Malleability: Non-malleable, cannot be hammered into thin sheets. Ductility: Non-ductile, cannot be drawn into wires. Brittleness: Mostly brittle in nature. Conductivity: Bad conductors of heat and electricity. Exception: Graphite (a form of carbon) is a good conductor of electricity. Melting and Boiling Points: Low melting and boiling points. Exception: Diamond and graphite have very high melting points. Sonorousness: Non-sonorous, do not produce ringing sound when struck. DO WE KNOW? (Science in Life) (NEP GUIDELINES) - Elements in Living Organisms
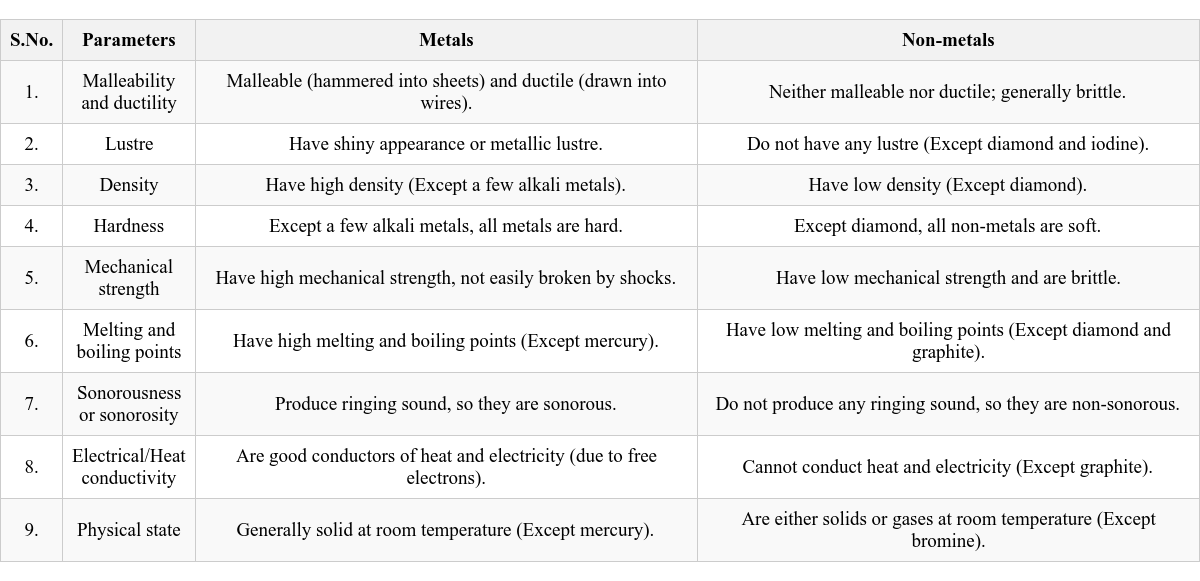
Most parts of all living organisms, including humans, are primarily composed of elements like carbon, hydrogen, and oxygen. Iron is found in haemoglobin, a respiratory pigment in blood. Magnesium is present in chlorophyll, the pigment responsible for photosynthesis in plants. Comparison: Metals vs. Non-metals
Here’s a summary of key differences between metals and non-metals:
3. Metalloids
Metalloids are elements that exhibit some properties of metals and some properties of non-metals. They are always solid. Examples include boron, arsenic, germanium, tellurium, polonium, silicon, and antimony.
4. Noble or Inert Gases
These are gaseous elements that do not react chemically with other elements. They are found in very small amounts, which is why they are also known as rare gases. Examples are helium, neon, argon, krypton, xenon, and radon.
Uses Of Elements
The unique properties of different elements make them incredibly useful in our daily lives.
Some common elements and their applications:
Copper and Aluminium: Used in electrical wires and utensils because they are good conductors of heat and electricity. Gold, Silver, and Platinum: Used in making jewellery due to their lustrous appearance. Iron: Used in making heavy tools and machines because of its strength. Mixed with zinc to produce brass. Mixed with tin to produce bronze. Both brass and bronze are used for statues, utensils, and machine parts. Diamonds: Used as gems in ornaments and for cutting glass due to their extreme hardness. Graphite: Used to make the “lead” in pencils. Argon or Neon: Filled in electric bulbs because they are inert and do not react with the tungsten filament, preserving the bulb’s life.
Representation Of Elements
The abbreviation used to represent an element is called a symbol.
Origin of Symbols of Elements
Ancient Scientists/Alchemists: Initially, alchemists used specific signs and symbols, often with mythological origins, to represent elements and compounds (Fig. 3.11).
Fig. 3.11 Alchemist’s symbols for elements Dalton’s Symbols of Elements: John Dalton was the first scientist to use systematic symbols. He represented atoms of various elements with circles containing different markings. Groups of these circles represented compounds.
Modern Symbols of the Elements
Modern symbols are based on rules for simplicity and universality:
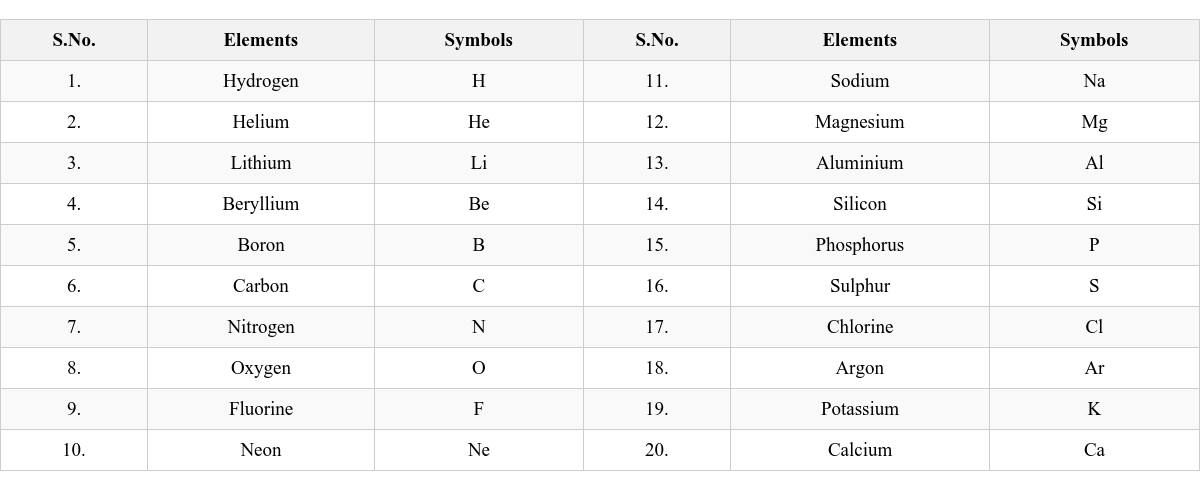
First Letter of English Name: The symbol is the first letter of the English name, written in capital. First Letter and Another Significant Letter of English Name: The first letter is capital, and the second (or third) significant letter is small. Calcium: Ca (first and second letter) Chlorine: Cl (first and third letter) Latin Names: Some symbols are derived from the element’s Latin name. Potassium: K (from Kalium) Symbols of First Twenty Elements
DO WE KNOW? (Science in Life) (NEP GUIDELINES)
Nitrogen is the most abundant element in the atmosphere, making up almost 78% of air gases. Oxygen is found in the greatest amount in the Earth’s crust, followed by silicon, aluminium, and iron. DO WE KNOW? (Science in Life) (NEP GUIDELINES)
The Periodic Table is an organized tabular arrangement of elements. It simplifies the study of elements by grouping them based on similar properties.
Compounds
Just as 26 letters of the English alphabet can form countless words, the approximately 118 different elements can combine in various ways to form a vast number of compounds.
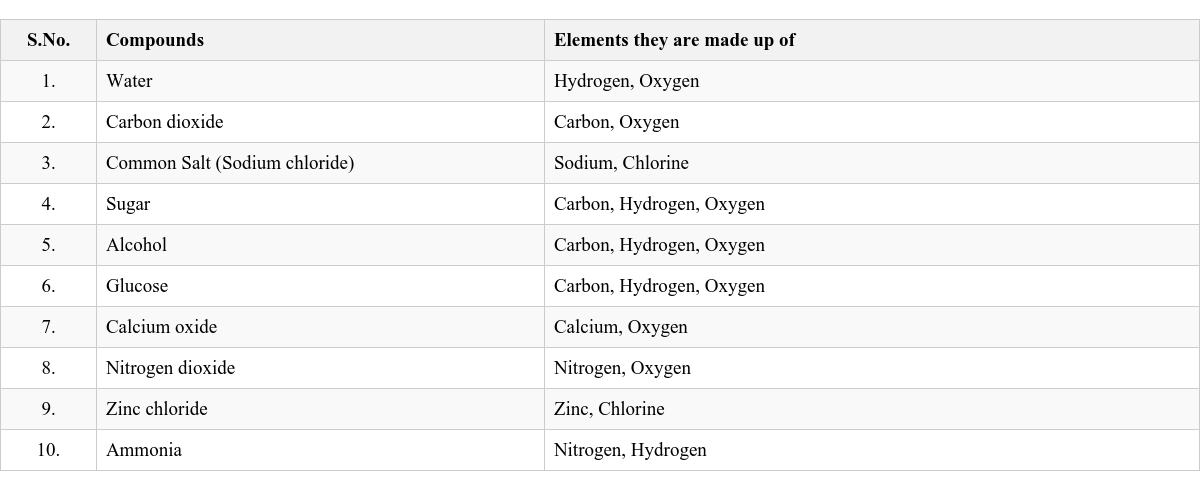
A compound is a pure substance made up of two or more elements chemically combined in a fixed proportion by mass. A molecule is the smallest unit of a compound that exhibits all the properties of that compound and has an independent existence. Examples of Compounds and their Constituent Elements
CIGEMES
The 117th element, Tennessine, was discovered in 2010. Recently, scientists created this super-heavy element by bombarding calcium ions onto a berkelium target. The formation of the 117th element is an important step towards producing and detecting other super-heavy elements.
Properties Of Compounds
Composition: A compound is formed from two or more elements and is always pure and homogeneous. Fixed Proportion: Elements combine in a definite proportion by mass to form a compound. Example: Water is formed from hydrogen and oxygen in a fixed ratio of 1:8 by mass. Some Common Compounds and Proportion of their Elements by Mass
3. Separation: The components of a compound cannot be separated by physical methods (like boiling, evaporation, filtration). They can only be separated by chemical methods.
* Example: Water can be broken down into hydrogen and oxygen through electrolysis (a chemical method).
4. Properties Difference: The physical and chemical properties of a compound are entirely different from the properties of its constituent elements.
* Example 1: Water
* Hydrogen (gas) burns, oxygen (gas) supports burning.
* Water (liquid) neither burns nor supports burning; it helps put out fires.
* Example 2: Carbon dioxide
* Carbon (solid) gives heat on burning, oxygen (gas) supports burning.
* Carbon dioxide (gas) does not support burning; it also helps put out fires.
* C(solid) + O₂(gas) → CO₂(gas)
* carbon + oxygen → carbon dioxide
5. Energy Change during Formation: Energy (in the form of heat or light) is either absorbed or released when a compound is formed.
* Example: When water is formed by burning hydrogen in oxygen, heat and light are released.
6. Molecular Similarity within Compound: All molecules of a specific compound are identical. However, molecules of one compound are different from molecules of another compound, meaning each compound has its own unique properties.
* Example: Water molecules are different from carbon dioxide molecules, leading to different properties.
Molecules of Elements
Atoms of most elements do not exist independently; noble gases are an exception. When two or more atoms of the same element combine, they form a molecule of an element.
An atom of oxygen does not exist independently. Two oxygen atoms combine to form one molecule of oxygen (O_2). Three oxygen atoms combine to form one molecule of ozone (O_3). Two nitrogen atoms combine to form one molecule of nitrogen (N_2). Atomicity: The number of atoms present in the molecule of an element.
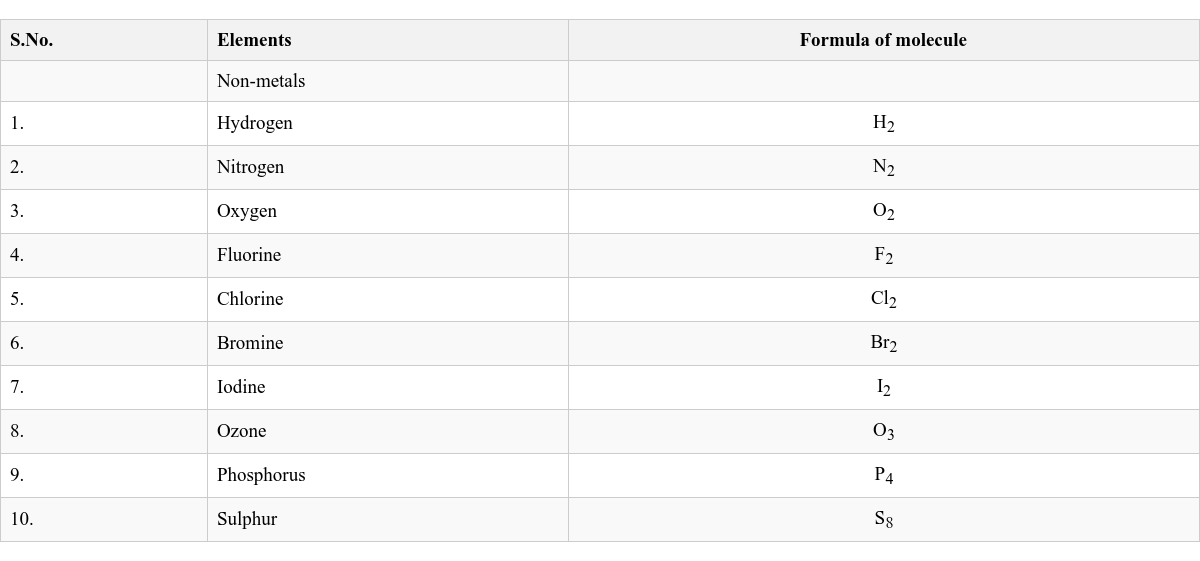
Based on atomicity, molecules can be classified as: Monoatomic Molecule: Contains one atom. Atomicity is one. Examples: Helium (He), Neon (Ne), Argon (Ar). Diatomic Molecule: Contains two atoms. Examples: Nitrogen (N_2), Oxygen (O_2), Hydrogen (H_2). Triatomic Molecule: Contains three atoms. Tetraatomic Molecule: Contains four atoms. Example: Phosphorus (P_4). Polyatomic Molecule: Contains more than four atoms. Example: Sulphur (S_8).
Molecules of Compounds
When atoms of two or more different elements combine chemically, they form a molecule of a compound.
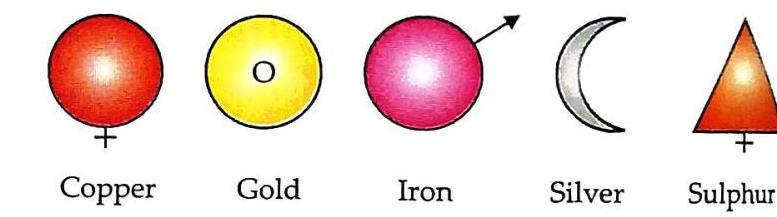
Two hydrogen atoms and one oxygen atom chemically combine to form a molecule of water (H_2O). One carbon atom and two oxygen atoms chemically combine to form a molecule of carbon dioxide (CO_2). One zinc atom and two chlorine atoms chemically combine to form a molecule of zinc chloride. One sodium atom and one chlorine atom chemically combine to form a molecule of sodium chloride. Diagrammatic Representation of Examples of Atoms and Molecules
Table 3.6 Diagrammatic representation of examples of atoms and molecules Uses Of Compounds
Compounds are essential in various aspects of life:
Water: A universal solvent, crucial for transporting dissolved substances in the body (via blood) and for plants taking minerals from soil. It’s also used in medical and industrial solutions. Carbon Dioxide: Used by plants for photosynthesis (making food). Sodium Chloride (Common Salt): Used in food to enhance taste.
Formula
A formula is a short, universally accepted way to represent the molecule of an element or a compound. Using formulas saves time, space, and energy.
The formula of a bromine molecule is Br_2. This indicates that two bromine atoms combine to form one bromine molecule. The formula of carbon dioxide is CO_2. This indicates that one carbon atom and two oxygen atoms combine to form one carbon dioxide molecule. Formulae Of Elements
The molecules of elements are represented by their formulas.
The formula for a diatomic oxygen molecule is O_2. ‘O’ is the symbol for the oxygen element. The subscript ‘2’ indicates that two atoms are present in one molecule of oxygen. The formula for a triatomic ozone molecule is O_3. ‘O’ is the symbol for oxygen. The subscript ‘3’ indicates that three atoms are present in one molecule of ozone. Important Distinction: O_2 and 2O are not the same and have different meanings:
O_2 represents one molecule of oxygen. 2O represents two separate atoms of oxygen. Formula of Molecules of Some Common Elements (Non-metals)
Formulae Of Compounds
Compounds are also represented by chemical formulas.
Water (H_2O): One molecule of water is formed by the chemical combination of two hydrogen atoms with one oxygen atom. Carbon Dioxide (CO_2): One molecule of carbon dioxide is formed by the chemical combination of one carbon atom with two oxygen atoms. Importance Of Chemical Formulae
A chemical formula provides valuable information about a compound:
Elements Present: It tells us which elements make up the compound. Number and Ratio of Atoms: It indicates the number of atoms of each element in one molecule and their precise ratio. Mass of One Molecule: It allows for the calculation of the mass of one molecule of the compound by adding the masses of its constituent atoms. Example 1: Carbon Dioxide (CO_2) Constituent elements: Carbon © and Oxygen (O). Atoms: One carbon atom and two oxygen atoms. Mass: Can be calculated by summing the mass of one carbon atom and two oxygen atoms. Example 2: Sodium Carbonate (Na_2CO_3)  Self Study
Self Study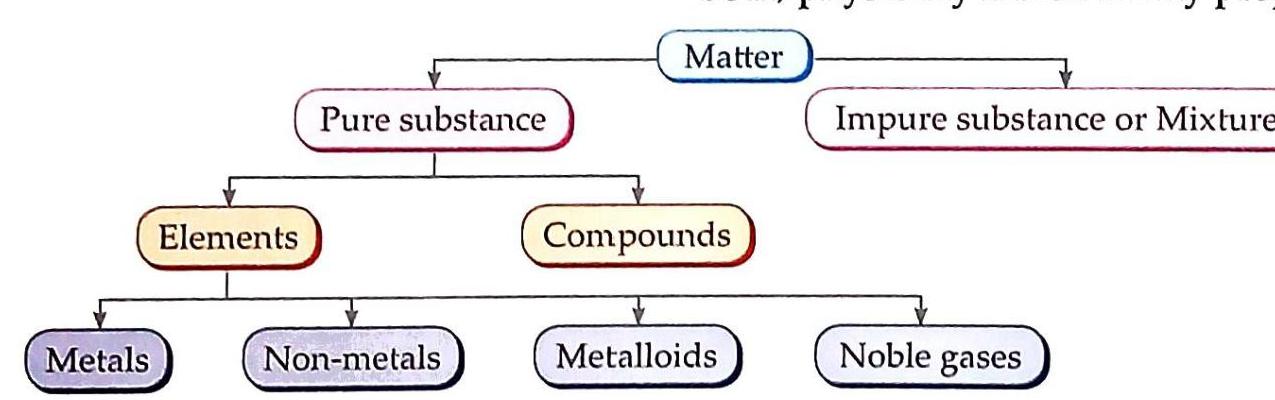





Fig. 3.4 Zinc
Fig. 3.4 Zinc











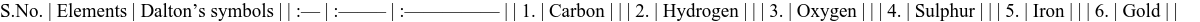



3. Separation: The components of a compound cannot be separated by physical methods (like boiling, evaporation, filtration). They can only be separated by chemical methods.
* Example: Water can be broken down into hydrogen and oxygen through electrolysis (a chemical method).
4. Properties Difference: The physical and chemical properties of a compound are entirely different from the properties of its constituent elements.
* Example 1: Water
* Hydrogen (gas) burns, oxygen (gas) supports burning.
* Water (liquid) neither burns nor supports burning; it helps put out fires.
* Example 2: Carbon dioxide
* Carbon (solid) gives heat on burning, oxygen (gas) supports burning.
* Carbon dioxide (gas) does not support burning; it also helps put out fires.
* C(solid) + O₂(gas) → CO₂(gas)
* carbon + oxygen → carbon dioxide
5. Energy Change during Formation: Energy (in the form of heat or light) is either absorbed or released when a compound is formed.
* Example: When water is formed by burning hydrogen in oxygen, heat and light are released.
6. Molecular Similarity within Compound: All molecules of a specific compound are identical. However, molecules of one compound are different from molecules of another compound, meaning each compound has its own unique properties.
* Example: Water molecules are different from carbon dioxide molecules, leading to different properties.
3. Separation: The components of a compound cannot be separated by physical methods (like boiling, evaporation, filtration). They can only be separated by chemical methods.
* Example: Water can be broken down into hydrogen and oxygen through electrolysis (a chemical method).
4. Properties Difference: The physical and chemical properties of a compound are entirely different from the properties of its constituent elements.
* Example 1: Water
* Hydrogen (gas) burns, oxygen (gas) supports burning.
* Water (liquid) neither burns nor supports burning; it helps put out fires.
* Example 2: Carbon dioxide
* Carbon (solid) gives heat on burning, oxygen (gas) supports burning.
* Carbon dioxide (gas) does not support burning; it also helps put out fires.
* C(solid) + O₂(gas) → CO₂(gas)
* carbon + oxygen → carbon dioxide
5. Energy Change during Formation: Energy (in the form of heat or light) is either absorbed or released when a compound is formed.
* Example: When water is formed by burning hydrogen in oxygen, heat and light are released.
6. Molecular Similarity within Compound: All molecules of a specific compound are identical. However, molecules of one compound are different from molecules of another compound, meaning each compound has its own unique properties.
* Example: Water molecules are different from carbon dioxide molecules, leading to different properties.


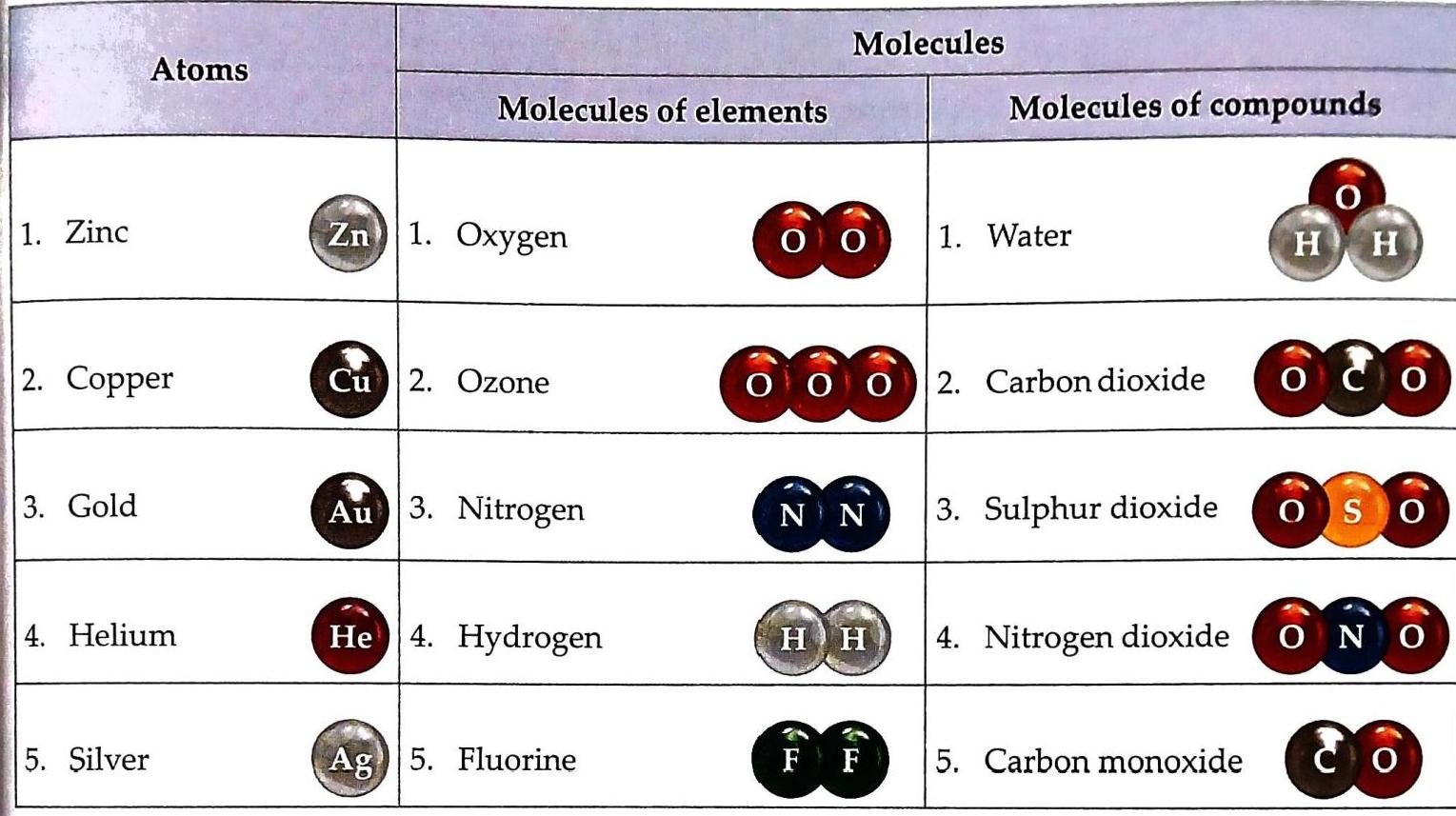
Table 3.6 Diagrammatic representation of examples of atoms and molecules
Table 3.6 Diagrammatic representation of examples of atoms and molecules