Chapter: 02. Matter
Chapter 2: Matter
When we look around, we observe a vast variety of things, such as plants, animals, water, rocks, buckets, chairs, tables, books, bags, pencils, erasers, shirts, shoes, cars, and bicycles. These things vary in color, shape, and size. Some are living, while others are non-living. They are also made of different materials; for example, a bucket is made of plastic, a chair from wood, and a bag from cloth.
A material is a substance from which something is made. Wood, plastic, and cloth are all examples of materials. All these materials share common characteristics: they possess mass and occupy space.
Anything that has mass and occupies space is called matter.
All living and non-living things, including our own bodies, the Sun, other stars, and planets, are composed of matter because they all have mass and occupy space. Additionally, substances that can be perceived by our senses are also considered matter. We can see, touch, feel, taste, or smell them. For example, while air cannot be seen, its presence can be felt, making air a form of matter.
It’s important to note that phenomena like heat, sound, shadow, and radio waves are not considered matter. This is because they are massless (without any mass) and do not occupy space.
DO WE KNOW? (Science in Life)
According to scientists, matter originated from a cosmic explosion known as the Big Bang. This event produced an immense amount of energy, which subsequently transformed into tiny particles of matter.
CLASSIFICATION OF MATTER
Matter can be broadly classified into two main types: living matter and non-living matter.
Fig. 2.1 Classification of matter 1. Living Matter
Definition: Matter that grows, moves, and reproduces on its own. Examples: All plants and animals. 2. Non-living Matter
Definition: Matter that does not grow, move, or reproduce on its own. Definition: Matter found in nature. Examples: Rock, water, air, wood. Definition: Matter that is created by humans. Examples: Plastic, steel, nylon, glass. Key Points on Materials and Things:
Different things are often made of different kinds of matter (e.g., a chair from wood, a bucket from plastic). The same kind of matter can be used to make many different things (e.g., wood used for a chair, table, and bed). A single thing can be made from different types of matter (e.g., a chair can be made of wood, plastic, or steel).
PROPERTIES OF MATTER
All types of matter share two fundamental common properties:
Matter Has Mass
The mass of a thing (object) is defined as the quantity or amount of matter contained within it.
Let’s explore activities to demonstrate that matter possesses mass.
Activity 1: Water and Stone Have Mass (Experiential Learning)
Aim: To show that water and stone have mass. Things Needed: Two glasses of the same size, a beam balance, water, and stones. Place two empty glasses of the same size on each pan of a beam balance. Observe that the balance is level.
(a) two empty glasses placed on a beam balance Pour water into one glass. Observe that the pan with the water-filled glass moves downwards.
(b) Pan with the glass containing water, moves downwards. Remove the water and place some stones in the same glass. Again, observe that the pan with the glass containing stones moves downwards.
© Pan with the glass containing stones, moves downwards. Observation: Both water and stones cause the pan to move downwards, indicating they have mass. The pan with the glass containing water moves downwards. The pan with the glass containing stones moves downwards. Activity 2: Air Has Mass (Experiential Learning)
Aim: To show that air has mass. Things Needed: Two balloons of the same size, a piece of string, a stick, and a needle. Inflate both balloons to the same size. Tie a string in the middle of a stick. Tie one balloon to each end of the stick. Balance the stick by adjusting the balloons until it is horizontal.
(a) balancing of inflated balloons (step 1) Carefully prick one of the balloons with a sharp needle.
(b) pricking of one balloon (step-2) Observation and Discussion: When the balloon is pricked, air escapes from the pinhole. The stick then tilts towards the still-inflated balloon, demonstrating that the air inside the inflated balloon has mass.
© The side with the inflated balloon moves down. Matter Occupies Space
The space occupied by matter is called its volume. Let’s perform activities to confirm that matter occupies space.
Activity 3: Stone Occupies Space (Experiential Learning)
Aim: To show that matter occupies space. Things Needed: A glass tumbler, a glass bowl, a stone, and a piece of thread. Place the glass tumbler inside the glass bowl. Fill the tumbler completely with water. Tie the stone with a thread and gently lower it into the water. Observe what happens. Remove the stone from the tumbler and observe the water level. When the stone is lowered into the water, some water overflows from the tumbler and collects in the bowl. After removing the stone, the water level in the tumbler decreases. Discussion: The stone takes up space, displacing the water, which causes it to overflow. When the stone is removed, the water level drops because the space it occupied is now empty. Activity 4: Air Occupies Space (Experiential Learning)
Aim: To show that air occupies space. Things Needed: A glass, water, and a vessel. Pour some water into a vessel. Quickly lower the glass into the water in an inverted (upside-down) position. Observe what happens.
Diagram (a) shows an inverted glass held vertically by a hand submerged in water within a beaker, with text indicating air cannot escape. Diagram (b) shows the inverted glass tilted, allowing air bubbles to escape from its rim into the water, with text indicating air escapes. Slightly tilt the glass while it is still submerged in the water. Observation and Discussion: When the inverted glass is lowered into water, water does not enter the glass, because the air inside the glass occupies the space. When the glass is tilted, water enters it as the air trapped inside escapes in the form of bubbles. This demonstrates that the seemingly “empty” glass was actually filled with air, which occupied space. Matter Offers Resistance
Another important property of matter is its ability to offer resistance.
Example 1: It is difficult to move against strong winds because air, a form of matter, offers resistance to your movement. Example 2: Similarly, swimming against a fast-flowing current in water is challenging because the water resists your motion.
COMPOSITION OF MATTER
The smallest particle that exhibits all the properties of matter and does not have an independent existence is called an atom. Two or more atoms join together to form molecules.
Molecule
The smallest particle of matter that exhibits all the properties of matter and has an independent existence is called a molecule.
Fig. 2.5 Composition of matter Atoms and molecules are incredibly small; they cannot be seen even with an ordinary microscope. A molecule can be made up of similar or different kinds of atoms. Example 1: Oxygen molecule Made up of two oxygen atoms.
Example 2: Water molecule Made up of two atoms of hydrogen and one atom of oxygen.
CHARACTERISTICS OF PARTICLES (MOLECULES) OF MATTER
The tiny particles (molecules) that make up matter possess several important characteristics:
Particles of matter are very small in size. Particles of matter are always in a continuous random motion. This motion occurs because the particles possess kinetic energy. The continuous zigzag motion of particles is known as Brownian motion. Robert Brown provided evidence for the existence of Brownian motion.
Fig. 2.6 Brownian motion Particles (molecules) of matter are held together by a force of attraction that exists between them. This force is called the interparticle or intermolecular force of attraction. Cohesive Force: The force of attraction between particles of the same kind. Adhesive Force: The force of attraction between particles of different kinds. Particles of matter have spaces between them. The space between the particles (molecules) of matter is called interparticle or intermolecular space. Activity 5: To observe intermolecular space (Experiential Learning)
Aim: To observe intermolecular space. Things Needed: A plastic beaker, some sugar, and pebbles. Fill three-fourths of a beaker with pebbles. Add some sugar to the beaker containing pebbles. Continue adding more sugar.
(a)
Fig. 2.7 Activity to show intermolecular space Observation and Discussion: The sugar gets accommodated within the spaces between the pebbles. This demonstrates that spaces exist between particles, which is analogous to intermolecular space between molecules of a substance. Activity 6: Intermolecular spaces in water (Experiential Learning)
Aim: To show that intermolecular spaces are present in water. Things Needed: A beaker, water, sugar, a spoon, and a glass rod. Pour some water into a beaker. Mark the initial level of the water.
Diagrams (a) and © show a beaker with water at an initial level. Sugar is depicted separately. Diagram (b) illustrates sugar dissolving in the water through stirring, resulting in a higher water level. Add a teaspoonful of sugar to the water and stir with a glass rod until it dissolves completely. Observe the change in the water level after the sugar has dissolved. Observation and Discussion: Initially, adding sugar might slightly raise the water level. However, once the sugar completely dissolves, the water level returns to its initial mark. This occurs because the sugar particles break down into smaller particles and occupy the intermolecular spaces that exist between the water molecules. Relationship between Intermolecular Force and Space
When the intermolecular force of attraction is maximum, the intermolecular space is minimum. As the intermolecular force of attraction decreases, the intermolecular space of matter increases.
STATES OF MATTER
Matter primarily exists in three main states: solid, liquid, and gas. The specific properties of a substance’s particles determine whether that substance exists as a solid, liquid, or gas.
These determining properties are:
(i) Intermolecular space: The distance between molecules.
(ii) Intermolecular force of attraction: The strength of the forces holding molecules together.
(iii) Movement of molecules: How freely molecules can move.
DO WE KNOW? (Science in Life)
Beyond solids, liquids, and gases, scientists have identified other states of matter. These include:
Plasma: Found in the Sun and other stars, responsible for their glow. Bose-Einstein Condensate (BEC) 1. Solid State
Molecular Arrangement in Solids
Fig. 2.9 Molecular arrangement in solids In solids, molecules are very closely packed, meaning the intermolecular space is negligible (very small). There is a strong intermolecular force of attraction, which holds the molecules in fixed positions. Molecules in a solid cannot move from one position to another; they can only vibrate about their fixed positions. As a result, molecules in solids are arranged in a definite manner. Properties of Solids
(i) Solids have a definite shape. Reason: Molecules are closely packed, and their positions are fixed. (ii) Solids have a definite volume. Reason: Intermolecular space is negligible. Activity 7: Solids have a definite shape and volume (Experiential Learning)
Aim: To show that solids have a definite shape and volume. Things Needed: A cup, a beaker, a small stone, a piece of thread, water, a big container, and a measuring cylinder. Place a small stone in a cup and observe its shape.  Self Study
Self Study

Fig. 2.1 Classification of matter
Fig. 2.1 Classification of matter
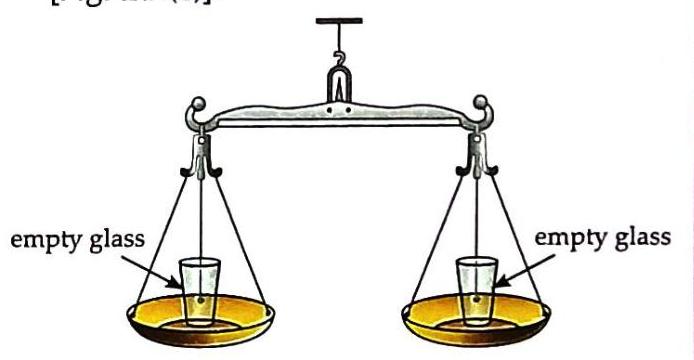
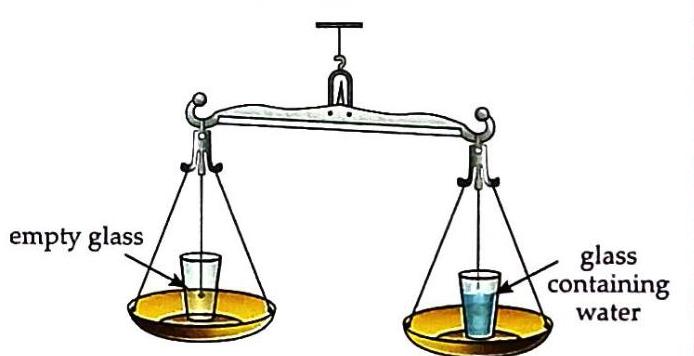
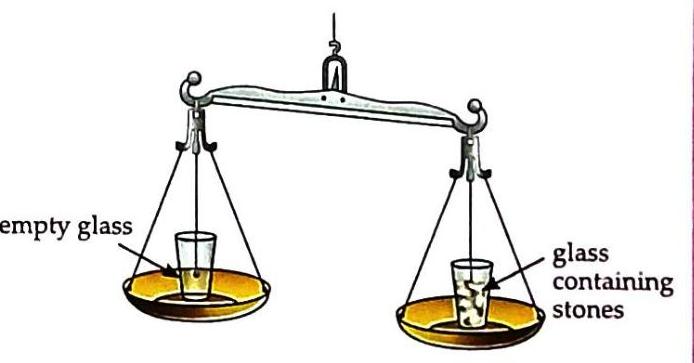
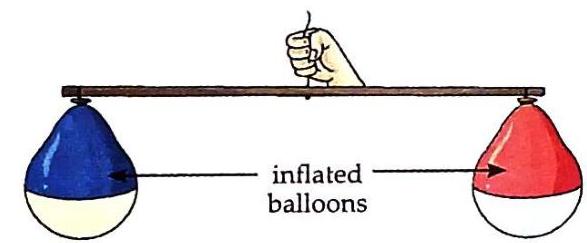
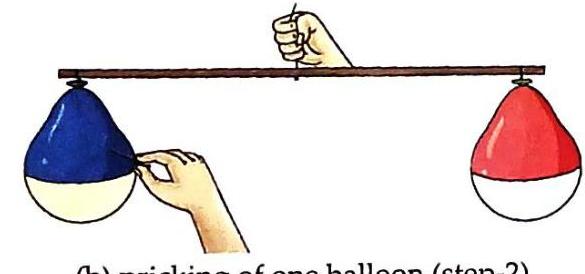
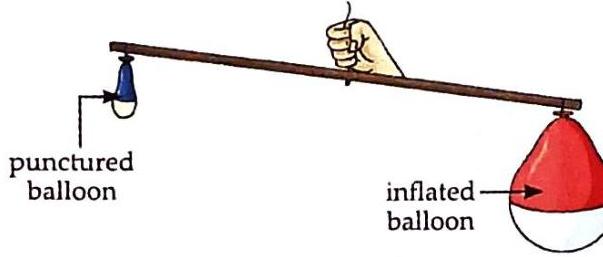
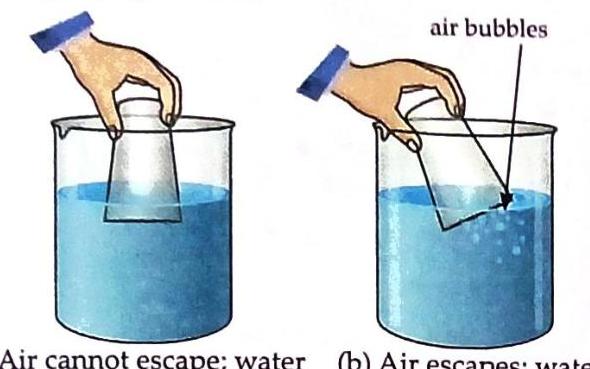
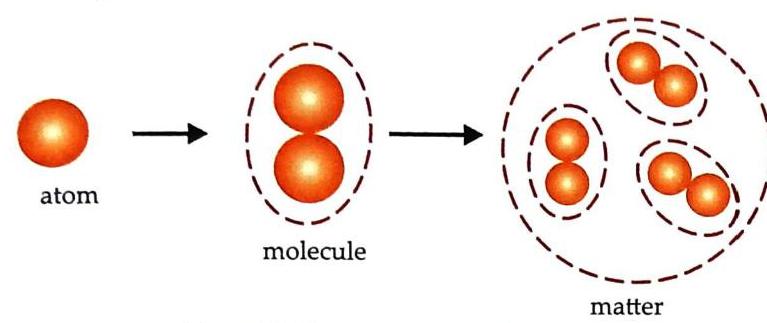
Fig. 2.5 Composition of matter
Fig. 2.5 Composition of matter
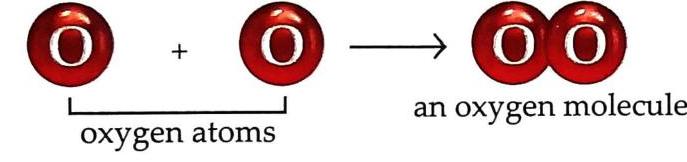
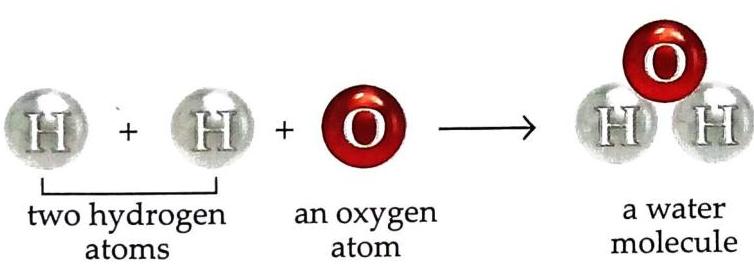
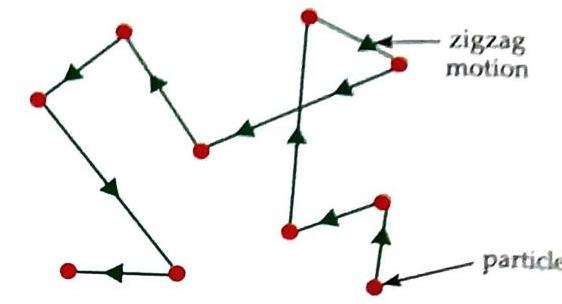
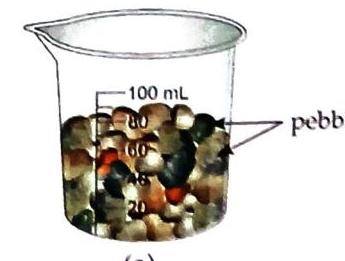
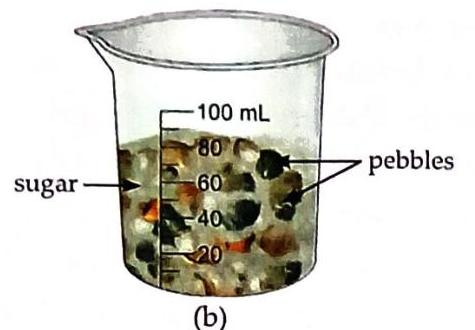
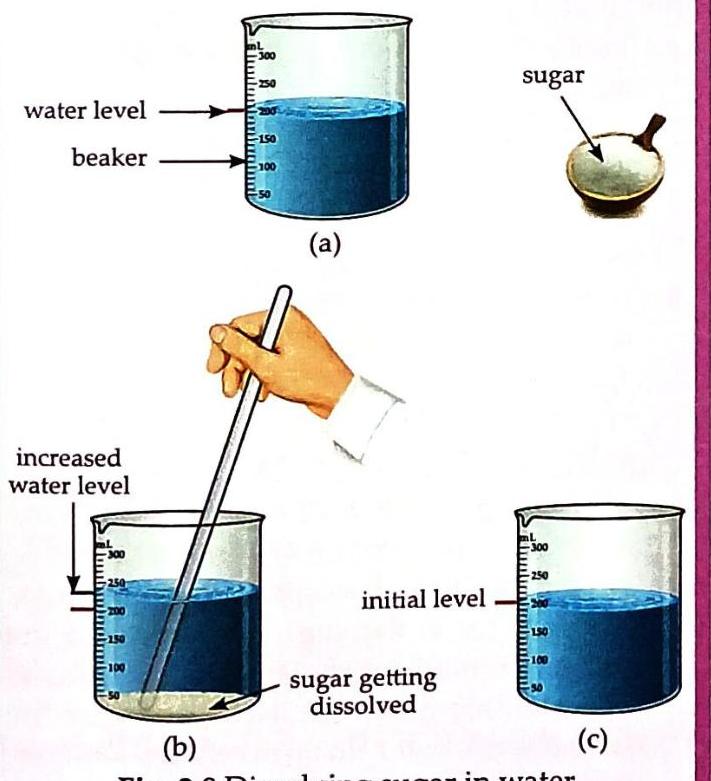
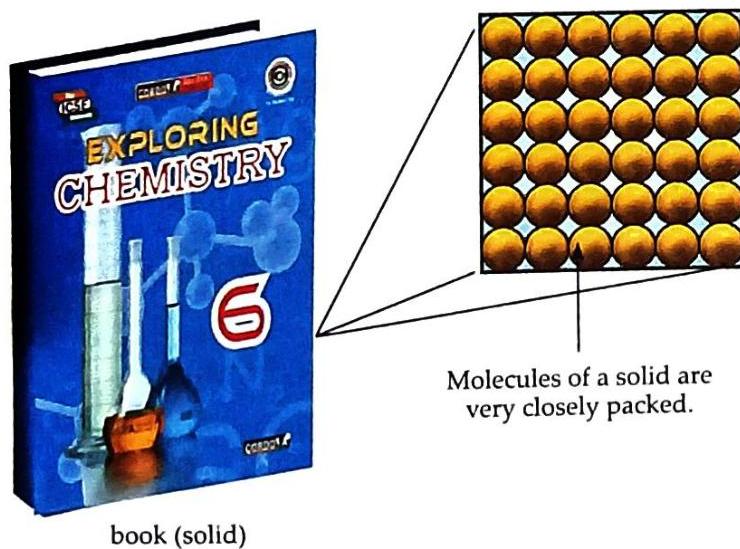
Fig. 2.9 Molecular arrangement in solids
Fig. 2.9 Molecular arrangement in solids