Chapter: 01. Introduction To Chemistry
Introduction to Chemistry
Since ancient times, humans have been curious about nature and their surroundings. This curiosity led to observations, conclusions, and the building of new ideas and concepts. These organized efforts are what we call Science.
What is Science?
Definition: Science is the systematic knowledge gained by humans through careful observations and experimentation. Scientist: A person who has vast knowledge of Science and is involved in scientific research. Branches of Science
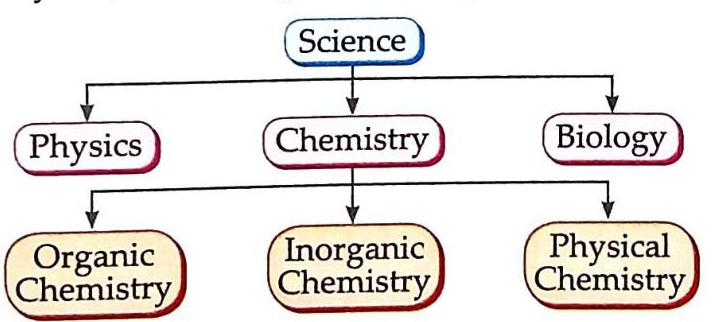
Science is broadly divided into three main branches:
Physics: Deals with energy and matter and how they relate to each other. Chemistry: Focuses on the composition and properties of matter and how it changes. Biology: The study of living organisms and life processes. Figure 1.1: Branches of Science
What is Chemistry?
When we look around us, we see many different things, or substances. Chemistry helps us answer important questions about these things:
What are these things made of? Do they stay the same forever, or do they change? What are their common properties? Are they useful to us, and in what ways? All substances are made up of matter. Chemistry is the branch of Science that studies matter and all its related aspects.
Definition of Chemistry
Chemistry: A branch of Science that deals with the study of the composition and the physical and chemical properties of various forms of matter. Chemistry in Everyday Life
Many things we use daily, like toothpastes, soaps, jams, and medicines, are made with the knowledge of Chemistry. Chemistry is involved in the making of almost everything around us. It explains various phenomena occurring around us, including chemical changes inside the body, such as digestion and respiration.
Development of Chemistry: A Historical Perspective
The journey of Chemistry into a modern science is deeply rooted in an ancient practice called Alchemy.
Alchemy
Definition: An ancient practice that was both scientific and spiritual. Alchemists: People who practiced alchemy. Methods: Their studies were based on trial and error, without standardized scientific methods. Contributions: They studied the nature of substances and created many useful compounds. The Goal of Alchemists
Philosopher’s Stone: Alchemists aimed to find a magical substance they called the philosopher’s stone. Beliefs about Philosopher’s Stone: Could turn iron and copper into gold when heated with them. Could extend one’s lifespan. Could help achieve immortality. Outcome: They did not succeed in finding this magical substance. However, their experiments led to the development of many important processes and substances that paved the way for modern Chemistry. Techniques Developed by Alchemists
Developed processes to extract metals from their ores. First to isolate zinc and phosphorus. Mixed different metals to form alloys. Example: Making fine quality bronze from tin and copper. Developed techniques for making glass, glass beads, artificial pearls, and gemstones. Developed dyes used in textiles and for painting palaces and tombs. Origins of Alchemy
Initially developed in Egypt and China. Indian Alchemists: Renowned for manufacturing: Transition to Modern Chemistry
By the 18th century, Chemistry emerged as a distinct science, separate from alchemy. Modern Chemistry owes a great deal to the foundational work and discoveries made by alchemists. Science Buzz!
The Iron Pillar of Delhi
Located in the Qutub Minar complex in Delhi. A 7-meter high structure made of iron. Contains a high percentage of phosphorus. Remarkably rust-resistant. Some Famous Scientists and Their Contributions to the Field of Chemistry
Many brilliant minds have shaped the field of Chemistry through their discoveries and theories.
Scientists and Their Major Contributions
Scientists and Their Discoveries
Importance of Chemistry
Chemistry is vital and plays a significant role in our daily lives. The progress of human civilization is greatly influenced by advancements in Chemistry. Chemistry is truly everywhere!
Figure 1.2: Importance of Chemistry in everyday life Let’s explore in detail how Chemistry contributes to various aspects of our lives.
1. Agriculture
Food is a basic human need. With the continuous increase in world population, there is a growing demand for food and a shortage of agricultural land. Chemistry helps farmers increase crop production.
Chemicals that provide nutrients to crops. Help increase agricultural production. Examples: Urea, sodium nitrate, potassium chloride, ammonium phosphate, calcium nitrate. Chemicals used to kill pests that harm crops and reduce yield. Chemicals used to kill insects. Examples: Heptachlor, BHC. Chemicals that protect crops from fungi. Example: Bordeaux mixture. 2. Food Processing and Preservation
The process of combining raw food ingredients using physical and chemical methods to create marketable food products. Involves chemical substances and various processes. Resulting products are ready for consumer use. Examples: Cheese, tinned vegetables, bread, jams, jelly, butter, snacks, soft drinks. The process of treating food with certain chemicals to prevent the growth of micro-organisms and spoilage. Preservatives: Chemicals used for food preservation. Examples: Salt (common since ancient times), benzoic acid, sorbic acid, sodium bisulphite. 3. Medicines
Medicines (Drugs): Chemical substances used to treat diseases and save lives. Chemists have synthesized a large number of medicines through extensive research. Types of Medicines and Their Effects
DO WE KNOW? (Science in Life)
Acidity: Excess acid in the stomach can cause indigestion and internal infections. Remedy: Taking mild bases like sodium bicarbonate and magnesium hydroxide can neutralize the excess acid and provide relief. Common Medicines: Penicillin, aspirin, paracetamol, tetracycline. 4. Clothing
Clothes protect our bodies from heat and cold. Natural Fibres (Older Use): Cotton, jute, silk, wool. Synthetic Fibres (Thanks to Chemistry): Chemistry opened the door to a world of new fibres. Examples: Nylon, terylene, polyester, rayon. Properties: Strong, wrinkle-resistant, dry quickly. Uses: Towels, bedsheets, bags, curtains, carpets, blankets, dress materials. 5. Cosmetics
Definition: Products used to clean, protect, and enhance the external appearance of the human body. Composed of various chemical compounds. Examples: Talcum powder, lipsticks, deodorants, body lotions, perfumes, bathing oil, skin care creams. Talcum Powder: A soft white powder made from talc (magnesium silicate), a mineral primarily composed of magnesium, silicon, and oxygen. 6. Industry
Chemistry has fueled the growth of many industries. It aids in the production of numerous products. Examples of industrial products thanks to Chemistry: Cleansing agents (soaps and detergents) 7. Building Materials
Raw materials for construction (buildings, bridges, roads) are products of Chemistry.  Self Study
Self Study




Iron pillar
Iron pillar


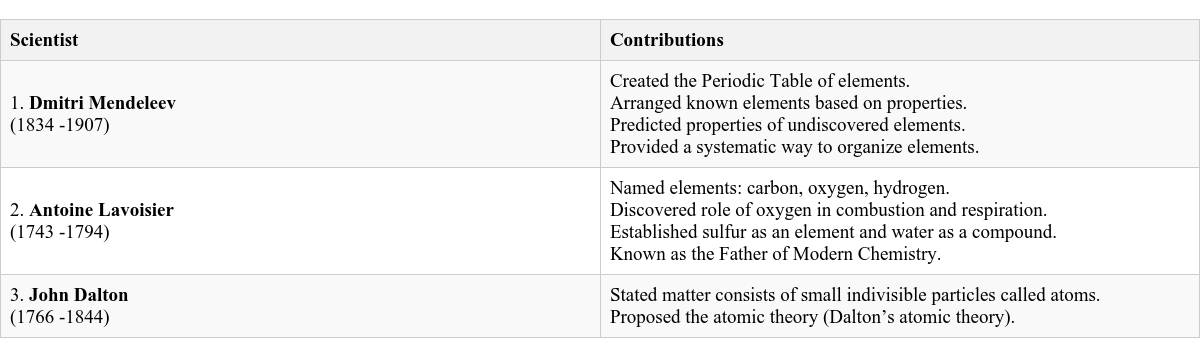

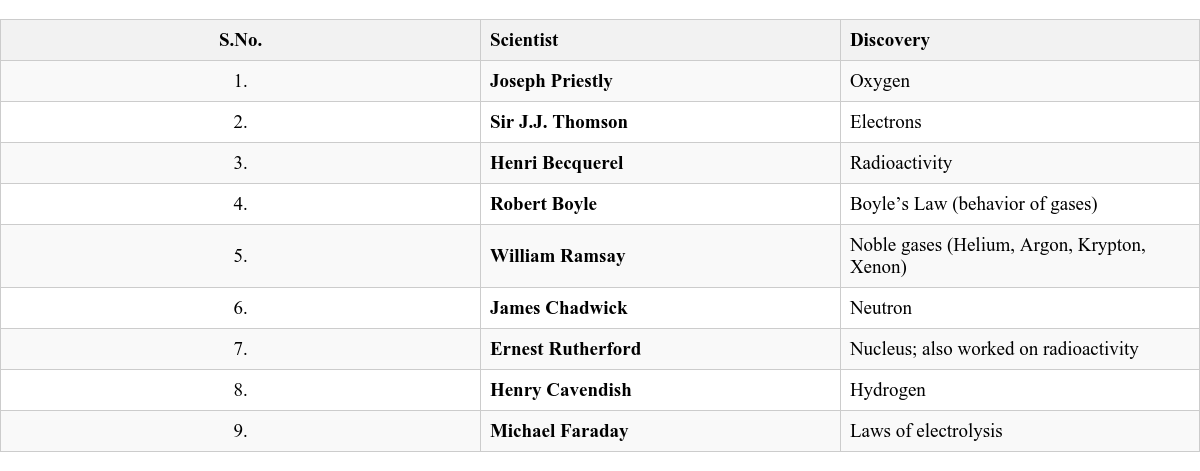


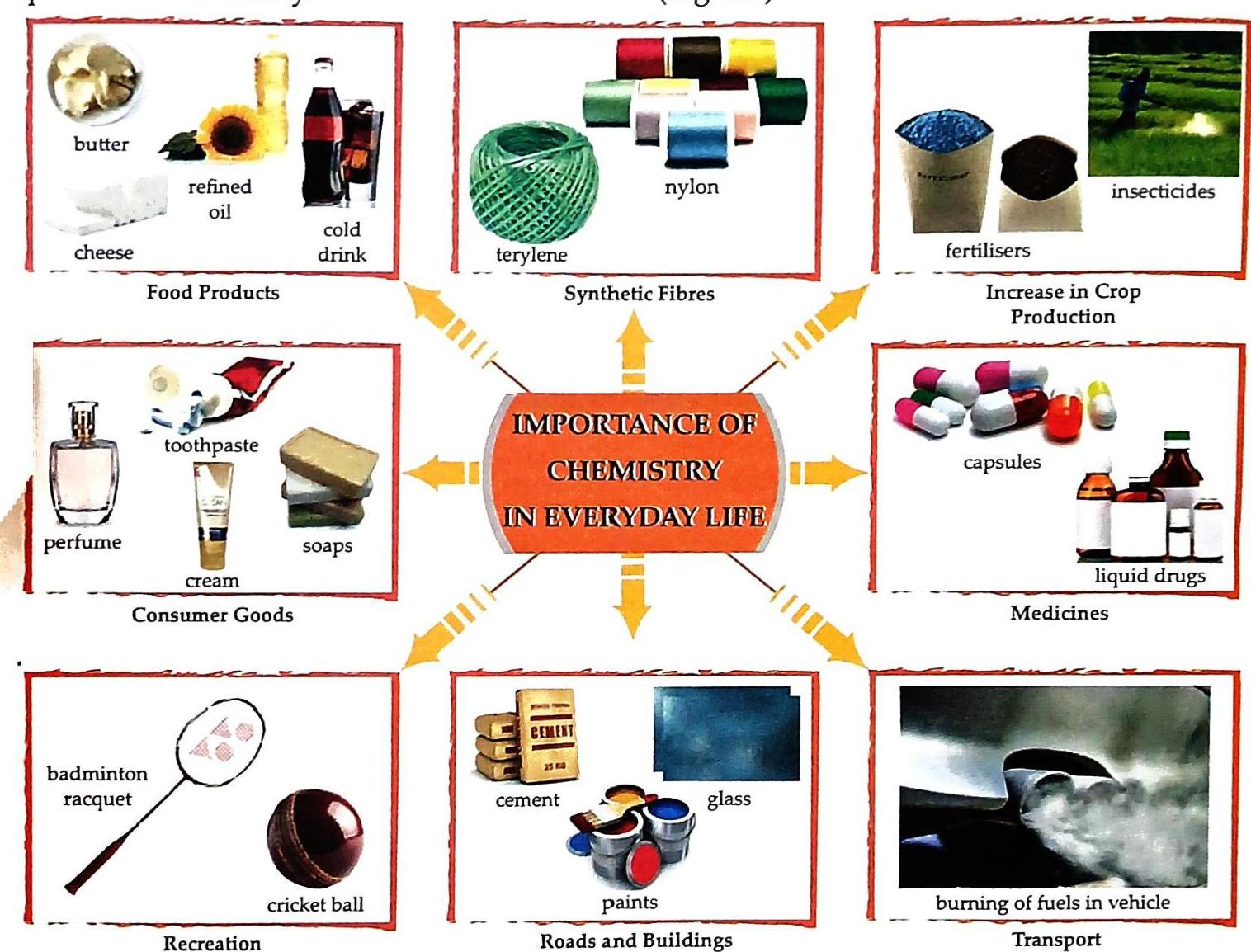
Figure 1.2: Importance of Chemistry in everyday life
Figure 1.2: Importance of Chemistry in everyday life