Skip to content
01. Matter
 Self Study
Self Study
Prepared by: learnloophq@gmail.com
Last edited 26 days ago by Learn LoopHQ.
Chapter: 01. Matter
Self-Study Document: Exploring Matter
LEARNING OUTCOMES
By the end of this study, you will be able to:
CONNECTING TO WHAT YOU ALREADY KNOW
Think about the things you see every day – the chair you sit on, the water you drink, the air you breathe. All these are examples of solids, liquids, or gases.
Consider these everyday situations:


INTRODUCTION: What is Matter?
Everything around us, whether it’s living or non-living, made by humans or found in nature, tiny or huge, falls into one of three categories: solids, liquids, or gases. The key features that make something “matter” are that it has mass and it occupies space.
What is Matter?
Matter is defined as anything that has mass and occupies space.
So, a book, the water you drink, and the air you breathe are all examples of matter because they have mass and take up space.
Two Common Properties of Matter:
It’s interesting to note that an object with a certain amount of matter can have more mass even if it takes up less space, and vice versa. For example:

An iron ball is heavier than a lump of cotton.
An iron ball is heavier than a lump of cotton.

Composition of Matter
Matter is made up of incredibly tiny particles that are too small to see even with a powerful microscope. These particles are called atoms and molecules.
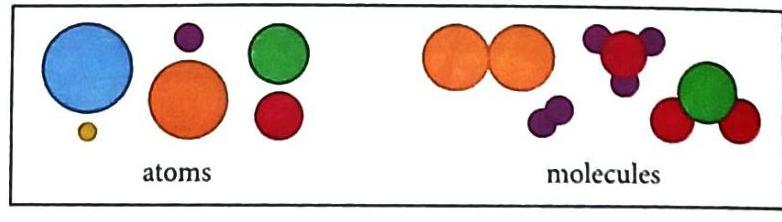
Atoms and molecules are the building blocks of matter.
Atoms and molecules are the building blocks of matter.

Key Vocabulary:
Understanding Forms of Energy vs. Matter
It’s a common misunderstanding to think that heat, light, and sound are forms of matter. However, this is not true!
STATES OF MATTER
You already know that things can be solids, liquids, or gases. These are known as the states of matter.
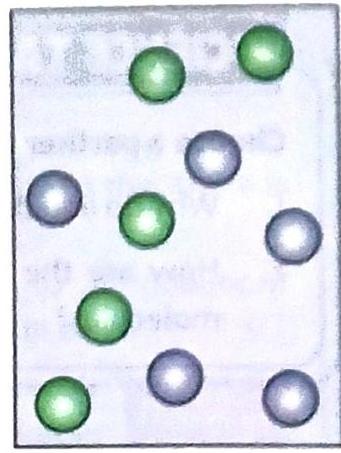
Matter, in all its states, is made up of tiny particles (atoms and molecules). These molecules are arranged differently in solids, liquids, and gases, and these arrangements determine their properties.
Understanding Particle Arrangement:
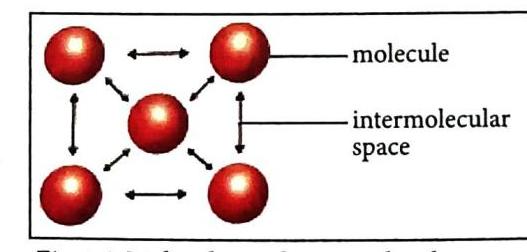
Molecules have space and attractive forces between them.
Molecules have space and attractive forces between them.

The Relationship between Space and Force:
The strength of the intermolecular force of attraction depends directly on the intermolecular space.
CHARACTERISTICS OF SOLIDS, LIQUIDS, AND GASES
Each state of matter has unique features based on how its molecules are arranged and how strongly they attract each other.
Solids
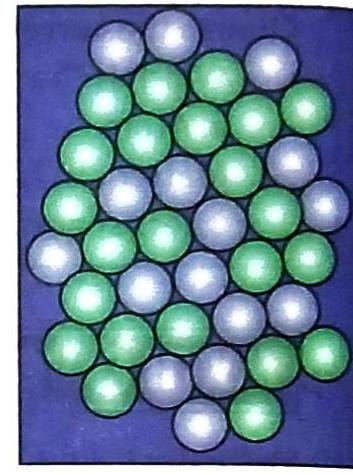
Reason Corner: Why is it easier to break a piece of coal than a piece of iron?
It’s easier to break a piece of coal than a piece of iron because the intermolecular forces of attraction in coal are weaker than those in iron. Iron has very strong intermolecular forces, making it much harder to break apart its molecules compared to coal.

Liquids
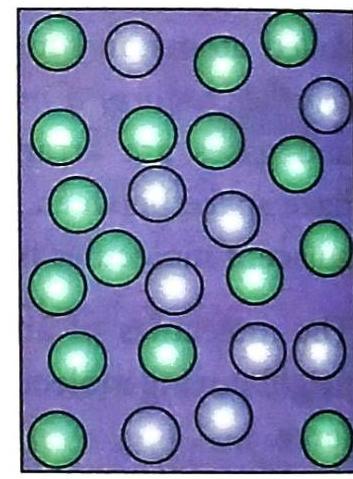
Gases
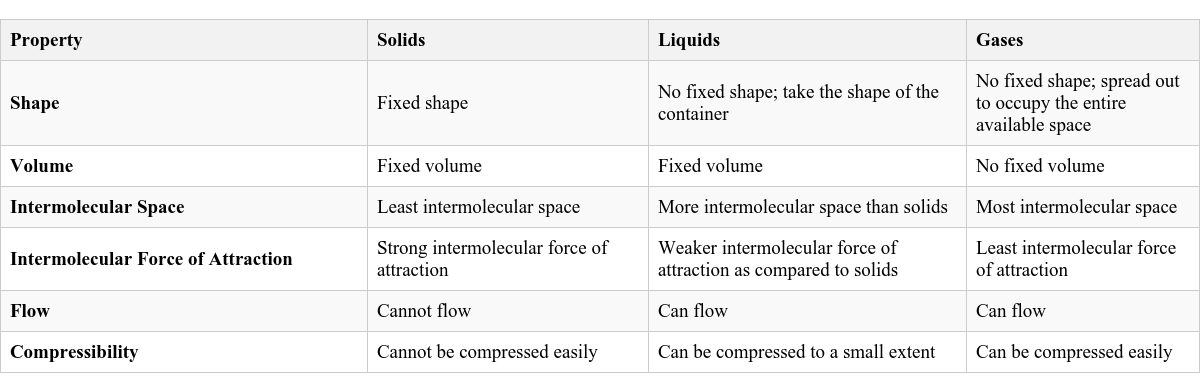
Summary of Distinguishing Properties
Here is a table summarizing the key differences between solids, liquids, and gases:

Beyond the Three States: Plasma
Sometimes, when a gas is heated to an extremely high temperature, it changes into a fourth state of matter called plasma. Plasma is found in lightning, stars, and neon signs!

Special Concepts and Connections
Diffusion
Diffusion is the spreading out and mixing of one substance with another substance. This happens because the particles of both substances are constantly moving.
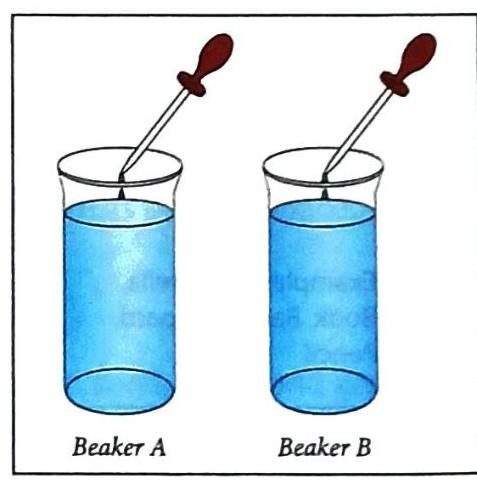
Ink diffusing in water at different temperatures.
Ink diffusing in water at different temperatures.


Environmental Connection: Air Pollution
Smoke from vehicles is a gas that spreads throughout the atmosphere, causing air pollution. Some places, like Matheran in Maharashtra (Asia’s only automobile-free hill station), have very little air pollution because there are no vehicles. This highlights how gases (pollutants) move and spread. Reducing air pollution involves careful planning towards resource efficiency and adapting to climate change, aligning with global goals for sustainable cities.

United Nations’ Sustainable Development Goal 11: Sustainable Cities and Communities.
United Nations’ Sustainable Development Goal 11: Sustainable Cities and Communities.


Demonstrating Gas Properties: Air Occupies Space
You can see that air, a gas, occupies space.
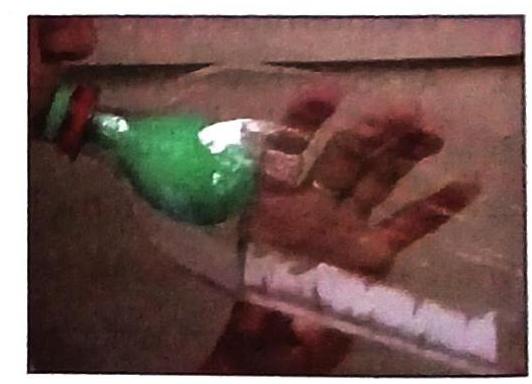
Experiment showing air occupies space.
Experiment showing air occupies space.


Science and Scientists: Democritus
The idea that matter is made of small particles is very old! Democritus (460 - 370 BCE), a famous Greek philosopher, proposed this theory.

Bust of Democritus.
Bust of Democritus.


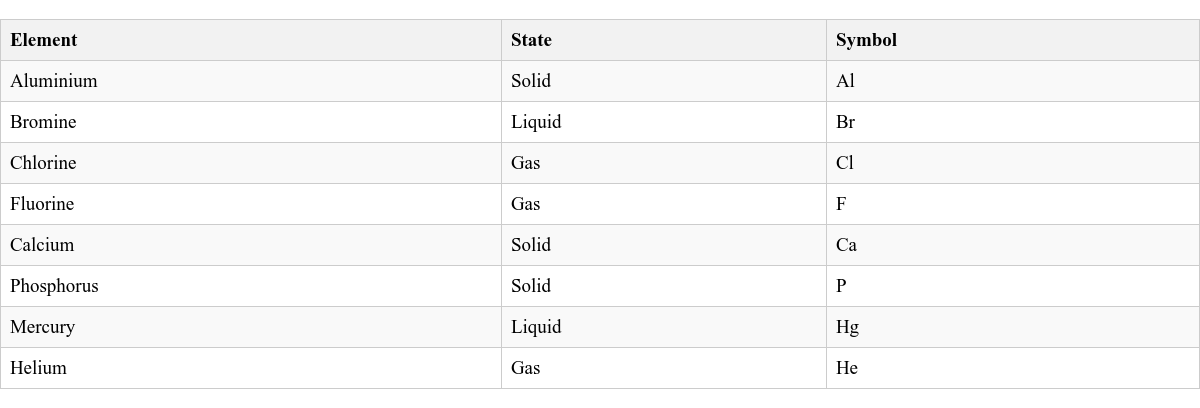
Integrating Knowledge: Elements and Their States
Elements are the basic building blocks of matter, and they can exist as solids, liquids, or gases at room temperature.
Here are some examples of elements and their common states:


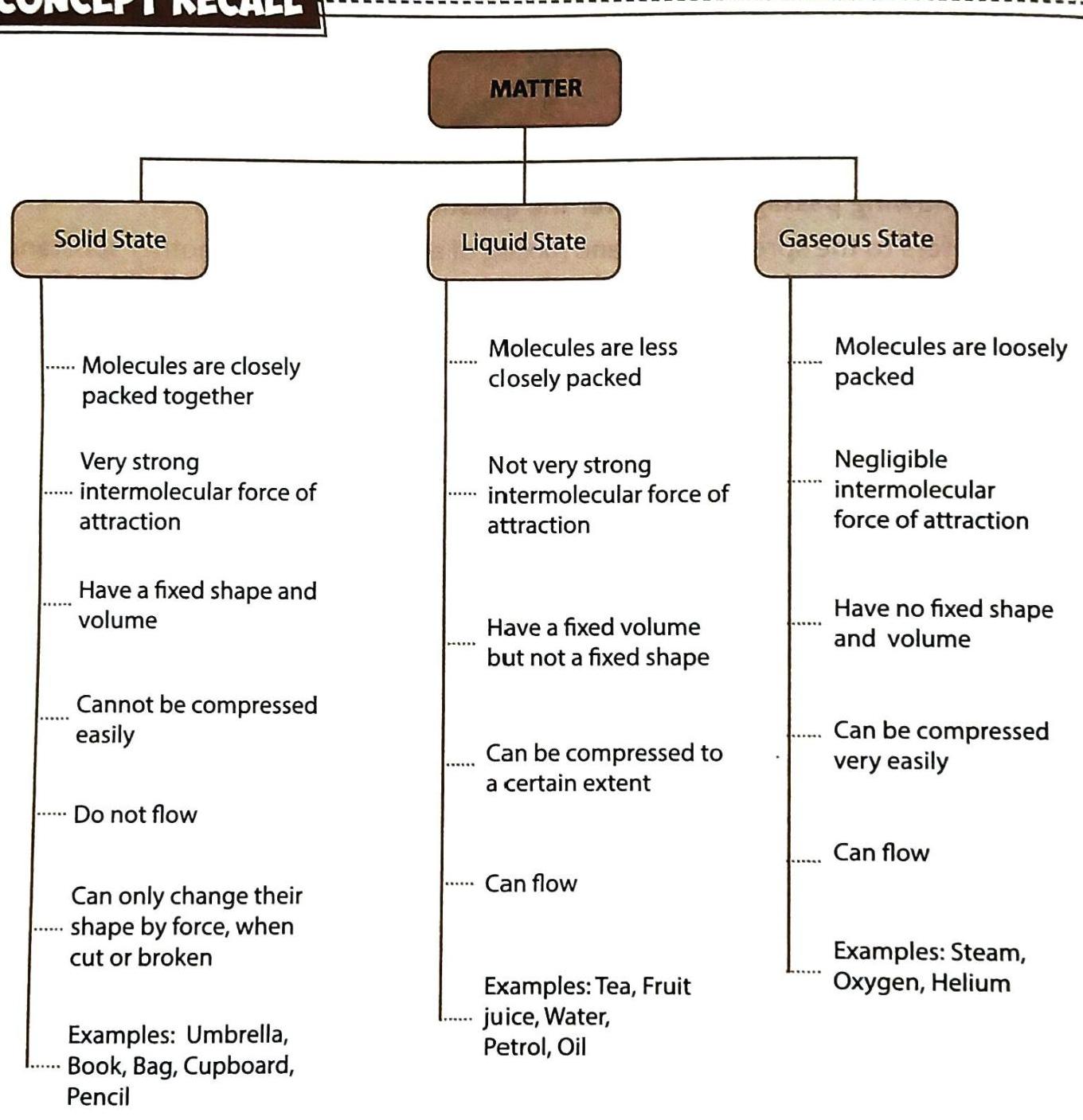
CONCEPT RECALL: Chapter Summary
Here’s a big picture view of everything you’ve learned about matter and its states:


Want to print your doc?
This is not the way.
This is not the way.
Try clicking the ⋯ next to your doc name or using a keyboard shortcut (
CtrlP
) instead.