Skip to content
Practice Sheets
 Practice Paper
Practice Paper
Prepared by: learnloophq@gmail.com
Last edited 26 days ago by Learn LoopHQ.
Chapter: 01. Matter
Matter - Practice Exam
Total Marks: [Suggest 30-40, depending on detail of answers]
Instructions:
Section A: Multiple Choice Questions (MCQs)
Choose the best answer for each question.
Section B: Fill in the Blanks
Complete the following sentences with the correct word(s).
Section C: True or False
Write ‘True’ if the statement is correct and ‘False’ if it is incorrect.
Section D: Short Answer Questions
Answer the following questions in one or two sentences.
Section E: Diagram-Based Questions
Observe the diagram and answer the questions that follow.
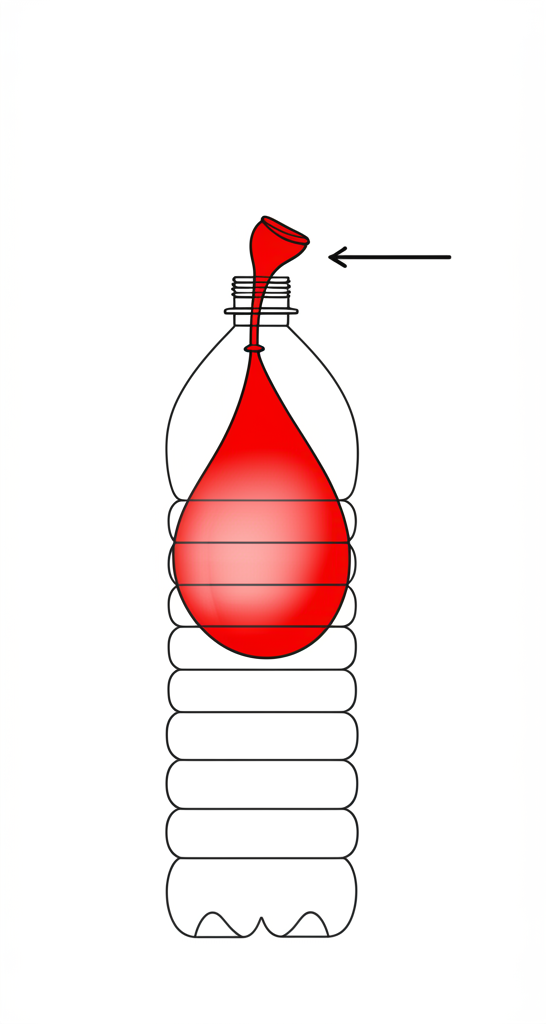
Image Description: A simple illustration showing a tightly sealed balloon inside a plastic bottle, with the balloon partially inflated but not filling the entire bottle. An arrow points from the balloon’s opening stretched over the bottle’s neck.
{
"image_description": "A tightly sealed balloon inside a plastic bottle, with the balloon partially inflated but not filling the entire bottle. An arrow points from the balloon's opening stretched over the bottle's neck.",
"style": "simple diagram, clear lines",
"elements_to_include": ["plastic bottle", "partially inflated balloon inside", "balloon opening over bottle neck", "arrow showing connection"],
"keywords_for_ai_generator": ["air occupies space experiment", "balloon in bottle diagram", "gas volume demonstration"]
}

Section F: Long Answer Questions
Answer the following questions in 4-5 sentences.
Want to print your doc?
This is not the way.
This is not the way.
Try clicking the ⋯ next to your doc name or using a keyboard shortcut (
CtrlP
) instead.