Chapter: 03. Elements And Compounds
Exercise For Revision
A. Write the symbols of the following:
B. Tick (✓) the correct options.
4. A/An ______ substance is a substance that contains same kind of particles (atoms or molecules).
* (b) pure
5. A/An ______ is a pure substance made up of only one kind of atoms that cannot be split into two or more simpler substances by any physical or chemical method.
* © Element
6. Which of the following is an element?
* (d) both (a) & (b)
7. Which of the following is not a metal?
* (d) bromine
8. Which of the following is easily cut with knife?
* (a) sodium
9. Particles of sugar are similar in shape, size and colour but this is not true in the case of soil because
* © soil is an impure substance
C. Complete the table given below:
Exercise For Revision A. Assertion-Reason based Questions.
Assertion: Water is a compound.
Reason: Water is formed by the chemical combination of hydrogen and oxygen in a fixed ratio. (a) Both A and R are true and R is the correct explanation of the assertion. Assertion: The formula for Phosphorous is P_4.
Reason: Atomicity of Phosphorous is 4. (a) Both A and R are true and R is the correct explanation of the assertion. B. Match the following.
C. State whether the following statements are true or false. Rewrite the false statements correctly.
A compound is a heterogeneous substance. False. A compound is a homogeneous substance. Properties of carbon dioxide are the same as those of carbon and oxygen. False. Properties of carbon dioxide are entirely different from those of carbon and oxygen. When two or more atoms of the same element combine, they form a molecule of an element. When atoms of two or more elements combine chemically, they form a molecule of a compound. Exercise For Revision
A. Tick (✓) the correct options.
Which of the following is the short way of representing a molecule of an element or a compound? The formula of a molecule of oxygen is O_2 and 20 are not same as B. Assertion-Reason based Questions.
4. Assertion: Diamond is used to cut glass.
Reason: Diamond is a form of carbon.
* (b) Both A and R are true but R is not the correct explanation of the assertion.
5. Assertion: Carbon dioxide is used to put off fires.
Reason: Carbon dioxide is a supporter of combustion.
* © A is true but R is false.
EXERCISE
Short Answer Questions:
Define metalloids, noble gases and atomicity. Metalloids: Elements that show some properties of metals and some properties of non-metals. Noble gases: Gaseous elements that do not react chemically with other elements. Atomicity: The number of atoms present in the molecule of an element. List any five uses of elements. Copper and aluminium metals are used in making electrical wires and utensils because they are good conductors of heat and electricity. Gold, silver and platinum are used in making jewellery because they are lustrous. Iron is used in making heavy tools and machines because it is strong. Diamonds are used as gems in ornaments and to cut glass because they are very hard. Argon or neon is filled in electric bulbs as they are inert and do not react with the tungsten filament. Give reason-
(a) Argon or neon is filled in electric bulbs. Argon or neon is filled in electric bulbs because they are noble (inert) gases and do not react with the tungsten filament of the bulb, thus preventing its oxidation and increasing its lifespan.
(b) Copper metal is used to make electric wires. Copper metal is used to make electric wires because it is an excellent conductor of electricity and is ductile, meaning it can be easily drawn into thin wires.
© A frying pan is made up of steel but its handle is made up of wood. A frying pan is made of steel (a metal alloy) because metals are good conductors of heat, allowing the pan to heat up quickly and cook food. Its handle is made of wood because wood is a bad conductor (insulator) of heat, preventing the hand from getting burnt when holding the hot pan. Classify the following elements as metals, non-metals and metalloids:
oxygen, arsenic, mercury, sodium, antimony, carbon, silicon, zinc, silver, nitrogen Metals: Mercury, Sodium, Zinc, Silver Non-metals: Oxygen, Carbon, Nitrogen Metalloids: Arsenic, Antimony, Silicon Long Answer Questions:
What are the properties of compounds? A compound is made up of two or more elements and is pure and homogeneous. A compound is formed by the combination of elements in a definite proportion by mass. The components of a compound cannot be separated by any physical method; they require chemical methods for separation. The physical and chemical properties of a compound are entirely different from the properties of its constituent elements. During the formation of a compound, energy (in the form of heat or light) is either absorbed or released. All the molecules of a compound are similar, and a particular compound has its own specific properties different from other compounds. List the steps of writing the formula of calcium oxide. What information do we get from a formula? Steps of writing the formula of Calcium Oxide: (This involves knowledge of valency which is usually taught later, but based on the provided tables, the formula is CaO. The chapter does not detail the steps of writing formulas from valencies explicitly but shows examples. If a detailed step-by-step is required, it implies pre-requisite knowledge of valency not fully covered.) Identify the constituent elements: Calcium (Ca) and Oxygen (O). (Implicit step based on common knowledge/further chapters): Calcium has a valency of 2, and Oxygen has a valency of 2. (Implicit step): The valencies are exchanged and simplified. Since both are 2, they cancel out to a 1:1 ratio. Write the symbols with the resulting subscripts: CaO. Information obtained from a formula: Elements present in the compound: For CaO, Calcium (Ca) and Oxygen (O). Number of atoms of each kind in one molecule and their ratio: For CaO, there is one atom of Calcium and one atom of Oxygen, in a ratio of 1:1. Mass of one molecule of the compound: This can be calculated by adding the mass of one atom of calcium and one atom of oxygen. In gold jewellery, you find that there is fine work of gold wires.
(a) Why is gold used in making jewellery? Gold is used in making jewellery because it is lustrous, attractive, and does not tarnish (react with air or water) easily, maintaining its shine over time.
(b) Which property of gold is used in making its wires? The property of ductility (ability to be drawn into thin wires) is used in making gold wires for jewellery. How are metals different from non-metals? (Refer to Table 3.1 in the main document for detailed differences. A summary of key differences is provided below.) Malleability and Ductility: Metals are malleable and ductile, while non-metals are neither (they are brittle). Lustre: Metals are shiny, while non-metals are generally dull (except iodine and diamond). Conductivity: Metals are good conductors of heat and electricity, while non-metals are generally bad conductors (except graphite). Physical State: Most metals are solid at room temperature (except mercury), while non-metals can be solid, liquid (bromine), or gas. Hardness: Metals are generally hard (except sodium and potassium), while non-metals are usually soft (except diamond). Application/Skill-based Questions:
How are molecules of elements different from molecules of compounds? Molecules of Elements: Formed when two or more atoms of the same element combine chemically. Example: O_2 (two oxygen atoms), N_2 (two nitrogen atoms). Molecules of Compounds: Formed when atoms of two or more different elements combine chemically in a fixed proportion. Example: H_2O (two hydrogen atoms and one oxygen atom), CO_2 (one carbon atom and two oxygen atoms). Observe the given picture (Fig. A) and answer the following questions:
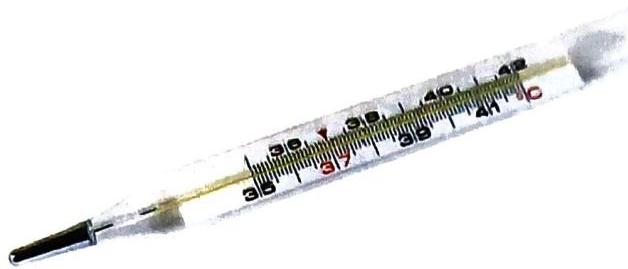
Fig. A
(a) What is shown here? For what purpose is it used? A clinical thermometer is shown here. It is used to measure body temperature.
(b) Name the substance filled inside it. Mercury.
© Is this substance a metal or non-metal? This substance is a metal. (It is the only metal that is liquid at room temperature.) An element is a pure substance made up of same kind of atoms. At present, nearly 118 elements are known but all of them do not occur free in nature, some of them have been synthesized by artificial methods. Based on their properties, they are mainly classified as metals and non-metals.
(a) Which of these non-metals is/are lustrous? (ii) Iodine
(b) Which element forms a triatomic molecule? (iv) oxygen (as ozone, O_3)  Answers to textbook exercises
Answers to textbook exercises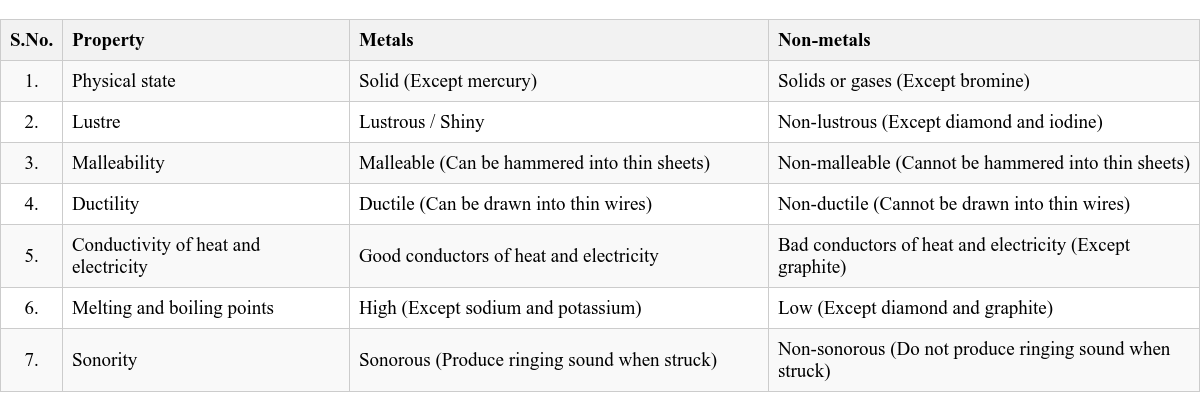
Exercise For Revision
Exercise For Revision