Chapter: 02. Matter
PROPERTIES OF MATTER
Q: Which one will have more mass- 1 kg cotton or 1 kg stone? Both 1 kg cotton and 1 kg stone have the same mass (1 kg). Mass refers to the quantity of matter, not its density or volume. Q: Why do balloons get inflated when we pump air inside them? Balloons get inflated because the air pumped inside them has mass and occupies space, pushing against the inner walls of the balloon. Q: Why does water flow out of the tumbler when we lower the stone in it? Water flows out of the tumbler when the stone is lowered into it because the stone occupies space, displacing the water that was previously in that space. Q: Briefly explain how air occupies space. Air occupies space because it is made of particles that take up volume. This is demonstrated when an inverted glass submerged in water prevents water from entering, as the air inside occupies that space, until the glass is tilted and the air escapes as bubbles. Exercise For Revision
A. Tick (✓) the correct options.
Which of the following is/are matter? Matter that grows, moves and reproduces on its own is called matter. Which of the following matter grow, move and reproduce on its own? Which of the following is not a property of matter? (d) It always exists as a liquid. B. Identify the odd one in each set and name the category to which the other three belong.
1. tiger glass
* Odd one: Glass. Category: Living Matter (assuming the context implies a set of living items with one non-living odd one out).
2. rock water
* (Note: With only two items, identifying an “odd one” and a category for “the other three” is ambiguous. Both rock and water are natural matter.)
3. plastic air
* Odd one: Air. Category: Human-made Matter (assuming the context implies a set of human-made items with one natural odd one out).
Exercise For Revision
A. Assertion-Reason based Question.
Assertion: When a substance changes its state from solid to liquid, its particles move more freely.
Reason: In the liquid state, the particles have more energy, resulting in lesser intermolecular force.
(a) Both A and R are true and R is the correct explanation of the assertion. B. Tick (✓) the correct options.
Two or more join together and form . A molecule is made up of kinds of atoms. The particles of matter are always . © in continuous random motion Which of the following states of matter has/have the strongest intermolecular force of attraction? Which of the following has a definite volume but no definite shape? A few substances are grouped in increasing order of their particles force of attraction. Which one of the following is a correct order? B. Case/Source-based Question.
Rohan’s mother took out a tray of ice cubes and left them on the kitchen slab. When she came back some time later, she found water in the tray. Explain why this happened.
This happened due to the process of melting. Ice, being in the solid state, absorbed heat energy from the warmer kitchen slab and the surrounding air. This increased the kinetic energy of its water molecules, causing them to vibrate more vigorously and overcome the strong intermolecular forces holding them in a fixed solid structure. As a result, the ice changed into its liquid state, water, at its melting point of 0°C. EXERCISE
Short Answer Questions:
List the characteristics of particles of matter. Particles of matter are very small in size. Particles of matter are always in a continuous random motion. Particles of matter are held together by a force of attraction (intermolecular force). Particles of matter have spaces between them (intermolecular space). List the properties that decide the state of matter. Intermolecular force of attraction What is meant by melting point and boiling point of a substance? Melting point: The fixed temperature at which a solid changes into its liquid state upon heating. Boiling point: The fixed temperature at which a liquid changes into its vapour state upon heating. What are the effects of heating on matter? Heating generally increases the kinetic energy of particles, leading to increased movement. It typically increases intermolecular space and decreases intermolecular force of attraction. Heating causes matter to expand. Heating can cause matter to change its state (e.g., melting, boiling, sublimation). Heating can cause chemical changes, forming new substances (e.g., burning). (a) Solids are rigid and retain their shape. Reason: Solids are rigid and retain their shape because their molecules are very closely packed, resulting in negligible intermolecular space and very strong intermolecular forces of attraction that hold the molecules in fixed positions. (b) Liquids and gases expand on heating. Reason: Liquids and gases expand on heating because heating increases the kinetic energy of their molecules, causing them to move more vigorously and further apart, thereby increasing the intermolecular space and overall volume. © A solid does not flow but a fluid flows. Reason: A solid does not flow because its molecules are held in fixed positions by strong intermolecular forces of attraction, preventing them from moving past one another. Fluids (liquids and gases) flow because their intermolecular forces of attraction are weaker (liquids) or negligible (gases), allowing their molecules to move freely and glide past each other or move randomly in all directions. Long Answer Questions:
Define matter. Write the common properties and composition of matter. Definition of Matter: Matter is anything that has mass and occupies space. Common Properties of Matter: Composition of Matter: Matter is composed of tiny particles called molecules, which are the smallest particles of matter with an independent existence. Molecules are, in turn, made up of even smaller particles called atoms, which are the smallest particles exhibiting matter’s properties but without independent existence. Atoms and molecules are too small to be seen with an ordinary microscope. What is intermolecular force of attraction? Differentiate between solids, liquids and gases on the basis of intermolecular force of attraction. Intermolecular Force of Attraction: This is the force of attraction that exists between the particles (molecules) of matter, holding them together. Differentiation based on Intermolecular Force of Attraction: Solids: Have a very strong intermolecular force of attraction, which tightly binds molecules in fixed positions. Liquids: Have a weak intermolecular force of attraction, allowing molecules to move freely within the liquid’s boundaries. Gases: Have a negligible (weakest) intermolecular force of attraction, allowing molecules to move absolutely freely and far apart. Observe the given figure and answer the following questions.
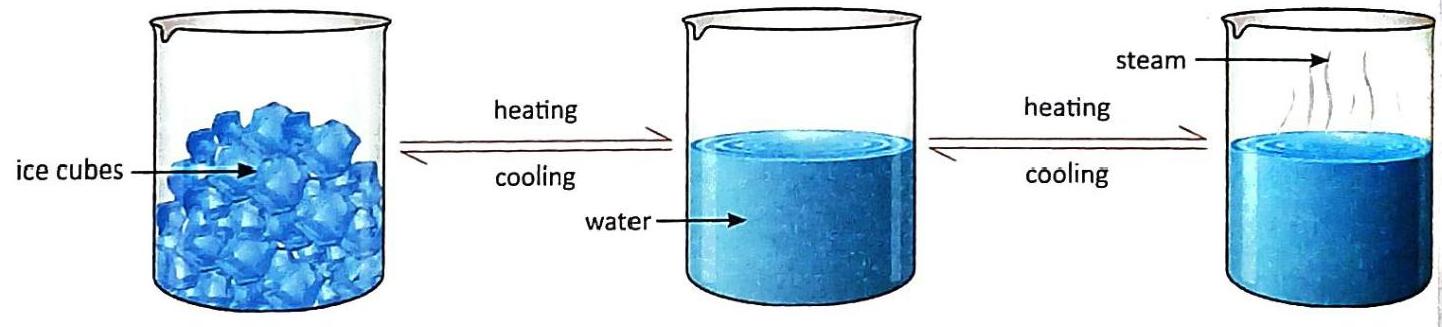
(a) What do you observe in fig. The figure illustrates the interconversion of states of water: solid (ice) to liquid (water) to gas (steam) upon heating, and gas (steam) to liquid (water) to solid (ice) upon cooling. (b) Why do ice cubes gets converted into water on heating? Ice cubes convert into water on heating due to melting. When heated, the water molecules in ice gain kinetic energy, vibrating more vigorously. This increased energy allows them to overcome the strong intermolecular forces holding them in a rigid solid structure, enabling them to move more freely and transition into the liquid state. How is the molecular arrangement of solids different from liquids and gases? Solids: Molecules are very closely packed in fixed, orderly positions with negligible intermolecular space. They only vibrate about these fixed positions. Liquids: Molecules are loosely packed compared to solids, with more intermolecular space. They are not in fixed positions and can move freely within the liquid’s boundaries, exhibiting no definite arrangement. Gases: Molecules are far apart from one another with very large intermolecular spaces. They move absolutely freely and randomly in all directions, having no definite arrangement. Application/Skill-based Questions:
Sakshi is cycling on the road. Suddenly, she observes that a tyre of her bicycle has punctured. What happens to the tube and the tyre of the bicycle when it gets punctured? When the bicycle tyre punctures, the highly compressed air inside the inner tube rapidly escapes through the hole. Since air is a gas, it expands to fill the larger available space of the atmosphere. As the air escapes, the internal pressure supporting the tyre’s shape is lost, causing the inner tube and the tyre to deflate or become flat. Observe the given pictures and answer the questions that follows.
Fig. A
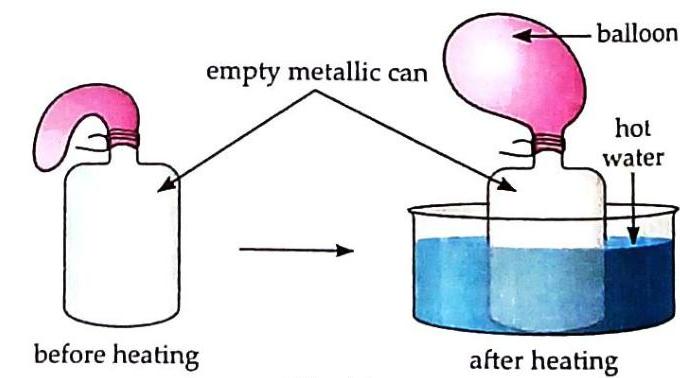
Fig. B (a) What do you observe in Fig. A? Name the process responsible for it. Observation: In Fig. A, blue crystals gradually dissolve and spread uniformly throughout the liquid, making the entire liquid blue. Process: This process is called dissolution (the dissolving of a solid in a liquid) or diffusion (the spreading of particles from an area of higher concentration to lower concentration). (b) What happens when the bottle shown in Fig. B is kept in cold water? Give reason for the same. When the bottle from Fig. B (with the inflated balloon) is kept in cold water, the balloon will deflate or shrink. This happens because the air inside the bottle contracts upon cooling. As the temperature drops, the gas molecules lose kinetic energy, move less vigorously, and occupy less volume, leading to a decrease in internal pressure that causes the balloon to collapse. © Explain the observation in Fig. B. In Fig. B, when the empty metallic can with a deflated balloon is heated in hot water, the balloon inflates. This demonstrates that gases expand on heating. The air inside the can absorbs heat, causing its molecules to gain kinetic energy, move faster, and spread further apart. This increased molecular activity and intermolecular space result in the air expanding and pushing against the balloon, causing it to inflate. Your mother is going to prepare tasty bread-jam for you. She takes out the jam bottle from the refrigerator, but is facing a problem opening the metallic cap of the jam bottle. How will you help her to open the cap of the jam bottle? [Hint: Solids expand on heating.] To help open the metallic cap, you should dip the metallic cap of the jam bottle in hot water for a few minutes. According to the principle that solids expand on heating, the metallic cap will expand slightly when heated. This expansion will loosen its grip on the glass jar, making it easier to twist and open.  Answers to textbook exercises
Answers to textbook exercises